Key Insights
The European neurology monitoring market, valued at €1.85 billion in 2025, is projected to experience robust growth, exhibiting a compound annual growth rate (CAGR) of 5.62% from 2025 to 2033. This expansion is driven by several key factors. The increasing prevalence of neurological disorders like stroke, traumatic brain injury (TBI), and epilepsy across Europe fuels demand for advanced monitoring technologies. Technological advancements, such as the development of miniaturized, portable, and wireless devices, are enhancing patient comfort and enabling remote monitoring capabilities, thereby contributing to market growth. Furthermore, rising healthcare expenditure and improved healthcare infrastructure in several European countries are creating a favorable environment for market expansion. The market is segmented by product type (MRI, EEG devices, cerebral oximeters, intracranial pressure monitors, and others) and by disease indication, with TBI, stroke, and sleep disorders representing significant segments. Germany, France, and the UK are expected to be leading markets within Europe, due to their advanced healthcare systems and substantial populations affected by neurological conditions. However, challenges such as high costs associated with advanced neurology monitoring equipment and the need for skilled professionals for operation and interpretation of data may pose some constraints to market growth.
The competitive landscape is characterized by a mix of established players like Philips Healthcare, Siemens Healthineers, and Medtronic, alongside smaller specialized companies. These companies are actively engaged in research and development, aiming to introduce innovative technologies to meet the growing needs of neurology monitoring. Strategic partnerships, mergers, and acquisitions are also expected to play a role in shaping the market dynamics in the coming years. The focus on improving diagnostic accuracy, reducing invasiveness, and enhancing patient outcomes will likely drive further innovation within the market. The increasing adoption of telehealth and remote patient monitoring solutions presents a significant opportunity for growth within the European neurology monitoring market, further fueling its expansion over the forecast period. Companies are focusing on developing user-friendly interfaces and integrating AI capabilities to improve data analysis and decision-making.
Europe Neurology Monitoring Industry Report: 2019-2033
This comprehensive report provides an in-depth analysis of the Europe Neurology Monitoring Industry, covering market size, growth drivers, key players, and future trends. With a study period spanning 2019-2033, a base year of 2025, and a forecast period of 2025-2033, this report offers invaluable insights for industry stakeholders, investors, and strategic decision-makers. The report includes detailed segmentation by product (Magnetic Resonance Imaging (MRI) Devices, Electroencephalography (EEG) Devices, Cerebral Oximeters, Intracranial Pressure Monitors, Other Products) and by disease (Traumatic Brain Injury (TBI), Stroke, Sleep Disorders, Parkinson's Disease, Epilepsy, Other Diseases), providing a granular understanding of market dynamics.
Europe Neurology Monitoring Industry Market Concentration & Dynamics
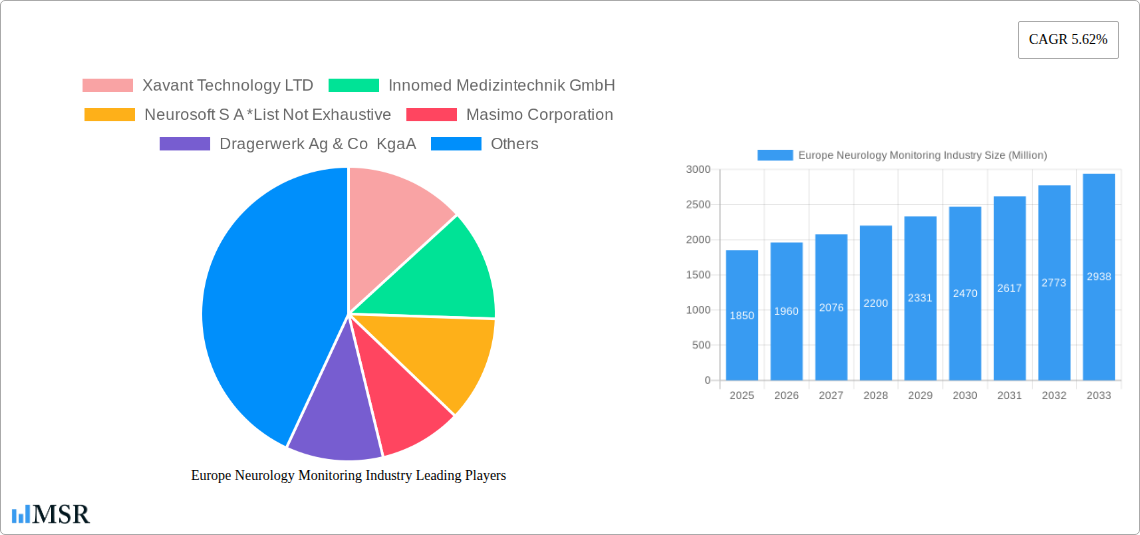
The Europe neurology monitoring market exhibits a moderately concentrated landscape, with a handful of multinational corporations holding significant market share. While precise market share figures for each company remain confidential, key players like Masimo Corporation, Drägerwerk AG & Co. KGaA, Philips Healthcare, Siemens Healthineers AG, and Medtronic PLC dominate the sector. Smaller, specialized companies like Xavant Technology LTD, Innomed Medizintechnik GmbH, and Neurosoft S A also contribute significantly, particularly in niche areas. The market is characterized by a dynamic innovation ecosystem, driven by continuous technological advancements in EEG, MRI, and other monitoring technologies.
Regulatory frameworks, primarily driven by the European Union's Medical Device Regulation (MDR), play a crucial role in shaping market dynamics. Stricter regulations increase compliance costs but simultaneously enhance patient safety and product quality. Substitute products, such as less technologically advanced monitoring methods, pose a limited threat due to the increasing preference for accurate and comprehensive neurology monitoring data. End-user trends indicate a growing demand for minimally invasive and wireless monitoring solutions, increasing the adoption rate of advanced technologies. M&A activity in the sector has been relatively moderate in recent years, with approximately xx deals recorded between 2019 and 2024, resulting in a xx% change in market concentration.
Europe Neurology Monitoring Industry Industry Insights & Trends
The Europe neurology monitoring market is experiencing robust growth, driven by several factors. The market size in 2025 is estimated at €xx Million, exhibiting a Compound Annual Growth Rate (CAGR) of xx% during the forecast period (2025-2033). This growth is fueled by increasing prevalence of neurological disorders like stroke and TBI across Europe. The aging population further contributes to the rise in demand for advanced monitoring solutions. Technological disruptions, such as the development of AI-powered diagnostic tools and wireless monitoring devices, significantly enhance the efficiency and accuracy of neurology monitoring, further driving market expansion. Evolving consumer behavior, characterized by increased health awareness and preference for personalized healthcare, also plays a crucial role in shaping market demand. Additionally, investments in research and development, coupled with favorable reimbursement policies in several European countries, further contribute to the market's growth trajectory.
Key Markets & Segments Leading Europe Neurology Monitoring Industry
While market data for specific regional and segment dominance is proprietary, several factors indicate key market trends. By product segment, EEG devices are likely to hold a larger market share due to their widespread use in various neurological conditions. MRI devices, while expensive, contribute significantly to the market revenue due to their importance in diagnosing complex neurological issues. Similarly, the high prevalence of stroke and TBI makes these disease segments the major revenue generators.
Growth Drivers (By Region): Germany, France, and the UK are likely to dominate due to their well-established healthcare infrastructure and higher prevalence of neurological disorders. Other countries with strong healthcare systems and economic development will also exhibit substantial growth.
Growth Drivers (By Product): Continuous technological innovations in EEG and MRI technology drive market growth. Development of portable and user-friendly cerebral oximeters and intracranial pressure monitors also expands market reach.
Growth Drivers (By Disease): The rising prevalence of age-related neurological disorders like stroke and Parkinson's disease is driving demand for effective monitoring solutions.
Europe Neurology Monitoring Industry Product Developments
Recent advancements have focused on miniaturization, wireless connectivity, and enhanced data analytics capabilities. The integration of AI and machine learning in EEG and MRI systems is improving diagnostic accuracy and efficiency. Products like NeuroCap (with its 22 pre-gelled electrodes), and the CE-marked MRI-compatible directSTIM DBS system exemplify this trend. These innovations offer significant competitive advantages by improving patient comfort, reducing healthcare costs, and enhancing diagnostic precision.
Challenges in the Europe Neurology Monitoring Industry Market
Regulatory hurdles, particularly navigating the complex MDR requirements, present a significant challenge for manufacturers. Supply chain disruptions, particularly concerning specialized components, can impact production and market availability. Furthermore, intense competition from established players and emerging companies creates pressure on pricing and profit margins. These factors collectively limit market growth and profitability to a degree.
Forces Driving Europe Neurology Monitoring Industry Growth
Technological advancements like AI-integrated devices and miniaturized sensors are major growth drivers. Increasing government funding for healthcare research and development fuels innovation. Favorable reimbursement policies in several European countries incentivize the adoption of advanced monitoring solutions. The increasing prevalence of neurological disorders and an aging population further boost demand.
Long-Term Growth Catalysts in the Europe Neurology Monitoring Industry
Long-term growth will be driven by continuous innovation in minimally invasive and wireless monitoring technologies. Strategic partnerships between technology companies and healthcare providers will enhance market penetration. Expansion into emerging markets within Europe and integration with other healthcare platforms will also contribute to future growth.
Emerging Opportunities in Europe Neurology Monitoring Industry
The integration of AI and machine learning for data analysis and improved diagnostics presents significant opportunities. The development of personalized medicine solutions tailored to specific neurological conditions offers substantial market potential. Expansion into remote patient monitoring will address the growing need for accessible and cost-effective healthcare.
Leading Players in the Europe Neurology Monitoring Industry Sector
- Xavant Technology LTD
- Innomed Medizintechnik GmbH
- Neurosoft S A
- Masimo Corporation
- Drägerwerk AG & Co. KGaA
- Natus Medical Inc
- Compumedics Limited
- Philips Healthcare
- Siemens Healthineers AG
- Medtronic PLC
- Advanced Brain Monitoring Inc
- Integra LifeSciences
- General Electronics (GE Healthcare)
- Nihon Kohden Corporation
Key Milestones in Europe Neurology Monitoring Industry Industry
- September 2022: Aleva Neurotherapeutics received CE mark approval for its MRI-compatible directSTIM DBS system, expanding its market reach and improving patient care.
- August 2022: Brain Scientific received CE mark approval for NeuroCap, enhancing the availability of advanced EEG technology for diagnosing stroke and epilepsy.
Strategic Outlook for Europe Neurology Monitoring Industry Market
The Europe neurology monitoring market presents significant growth potential, driven by technological advancements, increasing prevalence of neurological disorders, and a supportive regulatory environment. Strategic opportunities lie in developing innovative products, expanding into untapped markets, and fostering strategic partnerships to enhance market penetration. The focus on AI-powered diagnostics and personalized medicine will define the future of this dynamic industry.
Europe Neurology Monitoring Industry Segmentation
-
1. Product
- 1.1. Magnetic Resonance Imaging (MRI) Devices
- 1.2. Electroencephalography Devices
- 1.3. Cerebral Oximeters
- 1.4. Intracranial Pressure Monitors
- 1.5. Other Products
-
2. Disease
- 2.1. Traumatic Brain Injury (TBI)
- 2.2. Stroke
- 2.3. Sleep Disorders
- 2.4. Parkinson's Disease
- 2.5. Epilepsy
- 2.6. Other Diseases
Europe Neurology Monitoring Industry Segmentation By Geography
- 1. Germany
- 2. United Kingdom
- 3. France
- 4. Italy
- 5. Spain
- 6. Rest of Europe
Europe Neurology Monitoring Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 5.62% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increase in Incidence of Neurological Disorders; Growing Incidence of Traumatic Brain Injuries; Rise in the Aging Population
- 3.3. Market Restrains
- 3.3.1. High Cost of Equipment; Shortage of Trained Professionals
- 3.4. Market Trends
- 3.4.1. Parkinson's Disease Segment is Expected to Witness a Significant Growth During the Forecast Period.
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Product
- 5.1.1. Magnetic Resonance Imaging (MRI) Devices
- 5.1.2. Electroencephalography Devices
- 5.1.3. Cerebral Oximeters
- 5.1.4. Intracranial Pressure Monitors
- 5.1.5. Other Products
- 5.2. Market Analysis, Insights and Forecast - by Disease
- 5.2.1. Traumatic Brain Injury (TBI)
- 5.2.2. Stroke
- 5.2.3. Sleep Disorders
- 5.2.4. Parkinson's Disease
- 5.2.5. Epilepsy
- 5.2.6. Other Diseases
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. Germany
- 5.3.2. United Kingdom
- 5.3.3. France
- 5.3.4. Italy
- 5.3.5. Spain
- 5.3.6. Rest of Europe
- 5.1. Market Analysis, Insights and Forecast - by Product
- 6. Germany Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Product
- 6.1.1. Magnetic Resonance Imaging (MRI) Devices
- 6.1.2. Electroencephalography Devices
- 6.1.3. Cerebral Oximeters
- 6.1.4. Intracranial Pressure Monitors
- 6.1.5. Other Products
- 6.2. Market Analysis, Insights and Forecast - by Disease
- 6.2.1. Traumatic Brain Injury (TBI)
- 6.2.2. Stroke
- 6.2.3. Sleep Disorders
- 6.2.4. Parkinson's Disease
- 6.2.5. Epilepsy
- 6.2.6. Other Diseases
- 6.1. Market Analysis, Insights and Forecast - by Product
- 7. United Kingdom Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Product
- 7.1.1. Magnetic Resonance Imaging (MRI) Devices
- 7.1.2. Electroencephalography Devices
- 7.1.3. Cerebral Oximeters
- 7.1.4. Intracranial Pressure Monitors
- 7.1.5. Other Products
- 7.2. Market Analysis, Insights and Forecast - by Disease
- 7.2.1. Traumatic Brain Injury (TBI)
- 7.2.2. Stroke
- 7.2.3. Sleep Disorders
- 7.2.4. Parkinson's Disease
- 7.2.5. Epilepsy
- 7.2.6. Other Diseases
- 7.1. Market Analysis, Insights and Forecast - by Product
- 8. France Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Product
- 8.1.1. Magnetic Resonance Imaging (MRI) Devices
- 8.1.2. Electroencephalography Devices
- 8.1.3. Cerebral Oximeters
- 8.1.4. Intracranial Pressure Monitors
- 8.1.5. Other Products
- 8.2. Market Analysis, Insights and Forecast - by Disease
- 8.2.1. Traumatic Brain Injury (TBI)
- 8.2.2. Stroke
- 8.2.3. Sleep Disorders
- 8.2.4. Parkinson's Disease
- 8.2.5. Epilepsy
- 8.2.6. Other Diseases
- 8.1. Market Analysis, Insights and Forecast - by Product
- 9. Italy Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Product
- 9.1.1. Magnetic Resonance Imaging (MRI) Devices
- 9.1.2. Electroencephalography Devices
- 9.1.3. Cerebral Oximeters
- 9.1.4. Intracranial Pressure Monitors
- 9.1.5. Other Products
- 9.2. Market Analysis, Insights and Forecast - by Disease
- 9.2.1. Traumatic Brain Injury (TBI)
- 9.2.2. Stroke
- 9.2.3. Sleep Disorders
- 9.2.4. Parkinson's Disease
- 9.2.5. Epilepsy
- 9.2.6. Other Diseases
- 9.1. Market Analysis, Insights and Forecast - by Product
- 10. Spain Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Product
- 10.1.1. Magnetic Resonance Imaging (MRI) Devices
- 10.1.2. Electroencephalography Devices
- 10.1.3. Cerebral Oximeters
- 10.1.4. Intracranial Pressure Monitors
- 10.1.5. Other Products
- 10.2. Market Analysis, Insights and Forecast - by Disease
- 10.2.1. Traumatic Brain Injury (TBI)
- 10.2.2. Stroke
- 10.2.3. Sleep Disorders
- 10.2.4. Parkinson's Disease
- 10.2.5. Epilepsy
- 10.2.6. Other Diseases
- 10.1. Market Analysis, Insights and Forecast - by Product
- 11. Rest of Europe Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 11.1. Market Analysis, Insights and Forecast - by Product
- 11.1.1. Magnetic Resonance Imaging (MRI) Devices
- 11.1.2. Electroencephalography Devices
- 11.1.3. Cerebral Oximeters
- 11.1.4. Intracranial Pressure Monitors
- 11.1.5. Other Products
- 11.2. Market Analysis, Insights and Forecast - by Disease
- 11.2.1. Traumatic Brain Injury (TBI)
- 11.2.2. Stroke
- 11.2.3. Sleep Disorders
- 11.2.4. Parkinson's Disease
- 11.2.5. Epilepsy
- 11.2.6. Other Diseases
- 11.1. Market Analysis, Insights and Forecast - by Product
- 12. Germany Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 13. France Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 14. Italy Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 15. United Kingdom Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 16. Netherlands Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 17. Sweden Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 18. Rest of Europe Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 19. Competitive Analysis
- 19.1. Market Share Analysis 2024
- 19.2. Company Profiles
- 19.2.1 Xavant Technology LTD
- 19.2.1.1. Overview
- 19.2.1.2. Products
- 19.2.1.3. SWOT Analysis
- 19.2.1.4. Recent Developments
- 19.2.1.5. Financials (Based on Availability)
- 19.2.2 Innomed Medizintechnik GmbH
- 19.2.2.1. Overview
- 19.2.2.2. Products
- 19.2.2.3. SWOT Analysis
- 19.2.2.4. Recent Developments
- 19.2.2.5. Financials (Based on Availability)
- 19.2.3 Neurosoft S A *List Not Exhaustive
- 19.2.3.1. Overview
- 19.2.3.2. Products
- 19.2.3.3. SWOT Analysis
- 19.2.3.4. Recent Developments
- 19.2.3.5. Financials (Based on Availability)
- 19.2.4 Masimo Corporation
- 19.2.4.1. Overview
- 19.2.4.2. Products
- 19.2.4.3. SWOT Analysis
- 19.2.4.4. Recent Developments
- 19.2.4.5. Financials (Based on Availability)
- 19.2.5 Dragerwerk Ag & Co KgaA
- 19.2.5.1. Overview
- 19.2.5.2. Products
- 19.2.5.3. SWOT Analysis
- 19.2.5.4. Recent Developments
- 19.2.5.5. Financials (Based on Availability)
- 19.2.6 Natus Medical Inc
- 19.2.6.1. Overview
- 19.2.6.2. Products
- 19.2.6.3. SWOT Analysis
- 19.2.6.4. Recent Developments
- 19.2.6.5. Financials (Based on Availability)
- 19.2.7 Compumedics Limited
- 19.2.7.1. Overview
- 19.2.7.2. Products
- 19.2.7.3. SWOT Analysis
- 19.2.7.4. Recent Developments
- 19.2.7.5. Financials (Based on Availability)
- 19.2.8 Philips Healthcare
- 19.2.8.1. Overview
- 19.2.8.2. Products
- 19.2.8.3. SWOT Analysis
- 19.2.8.4. Recent Developments
- 19.2.8.5. Financials (Based on Availability)
- 19.2.9 Siemens Healthineers AG
- 19.2.9.1. Overview
- 19.2.9.2. Products
- 19.2.9.3. SWOT Analysis
- 19.2.9.4. Recent Developments
- 19.2.9.5. Financials (Based on Availability)
- 19.2.10 Medtronic PLC
- 19.2.10.1. Overview
- 19.2.10.2. Products
- 19.2.10.3. SWOT Analysis
- 19.2.10.4. Recent Developments
- 19.2.10.5. Financials (Based on Availability)
- 19.2.11 Advanced Brain Monitoring Inc
- 19.2.11.1. Overview
- 19.2.11.2. Products
- 19.2.11.3. SWOT Analysis
- 19.2.11.4. Recent Developments
- 19.2.11.5. Financials (Based on Availability)
- 19.2.12 Integra LifeSciences
- 19.2.12.1. Overview
- 19.2.12.2. Products
- 19.2.12.3. SWOT Analysis
- 19.2.12.4. Recent Developments
- 19.2.12.5. Financials (Based on Availability)
- 19.2.13 General Electronics (GE Healthcare)
- 19.2.13.1. Overview
- 19.2.13.2. Products
- 19.2.13.3. SWOT Analysis
- 19.2.13.4. Recent Developments
- 19.2.13.5. Financials (Based on Availability)
- 19.2.14 Nihon Kohden Corporation
- 19.2.14.1. Overview
- 19.2.14.2. Products
- 19.2.14.3. SWOT Analysis
- 19.2.14.4. Recent Developments
- 19.2.14.5. Financials (Based on Availability)
- 19.2.1 Xavant Technology LTD
List of Figures
- Figure 1: Europe Neurology Monitoring Industry Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Europe Neurology Monitoring Industry Share (%) by Company 2024
List of Tables
- Table 1: Europe Neurology Monitoring Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Europe Neurology Monitoring Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 3: Europe Neurology Monitoring Industry Revenue Million Forecast, by Disease 2019 & 2032
- Table 4: Europe Neurology Monitoring Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 5: Europe Neurology Monitoring Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 6: Germany Europe Neurology Monitoring Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 7: France Europe Neurology Monitoring Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 8: Italy Europe Neurology Monitoring Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 9: United Kingdom Europe Neurology Monitoring Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 10: Netherlands Europe Neurology Monitoring Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 11: Sweden Europe Neurology Monitoring Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 12: Rest of Europe Europe Neurology Monitoring Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 13: Europe Neurology Monitoring Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 14: Europe Neurology Monitoring Industry Revenue Million Forecast, by Disease 2019 & 2032
- Table 15: Europe Neurology Monitoring Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 16: Europe Neurology Monitoring Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 17: Europe Neurology Monitoring Industry Revenue Million Forecast, by Disease 2019 & 2032
- Table 18: Europe Neurology Monitoring Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 19: Europe Neurology Monitoring Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 20: Europe Neurology Monitoring Industry Revenue Million Forecast, by Disease 2019 & 2032
- Table 21: Europe Neurology Monitoring Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 22: Europe Neurology Monitoring Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 23: Europe Neurology Monitoring Industry Revenue Million Forecast, by Disease 2019 & 2032
- Table 24: Europe Neurology Monitoring Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 25: Europe Neurology Monitoring Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 26: Europe Neurology Monitoring Industry Revenue Million Forecast, by Disease 2019 & 2032
- Table 27: Europe Neurology Monitoring Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 28: Europe Neurology Monitoring Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 29: Europe Neurology Monitoring Industry Revenue Million Forecast, by Disease 2019 & 2032
- Table 30: Europe Neurology Monitoring Industry Revenue Million Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Europe Neurology Monitoring Industry?
The projected CAGR is approximately 5.62%.
2. Which companies are prominent players in the Europe Neurology Monitoring Industry?
Key companies in the market include Xavant Technology LTD, Innomed Medizintechnik GmbH, Neurosoft S A *List Not Exhaustive, Masimo Corporation, Dragerwerk Ag & Co KgaA, Natus Medical Inc, Compumedics Limited, Philips Healthcare, Siemens Healthineers AG, Medtronic PLC, Advanced Brain Monitoring Inc, Integra LifeSciences, General Electronics (GE Healthcare), Nihon Kohden Corporation.
3. What are the main segments of the Europe Neurology Monitoring Industry?
The market segments include Product, Disease.
4. Can you provide details about the market size?
The market size is estimated to be USD 1.85 Million as of 2022.
5. What are some drivers contributing to market growth?
Increase in Incidence of Neurological Disorders; Growing Incidence of Traumatic Brain Injuries; Rise in the Aging Population.
6. What are the notable trends driving market growth?
Parkinson's Disease Segment is Expected to Witness a Significant Growth During the Forecast Period..
7. Are there any restraints impacting market growth?
High Cost of Equipment; Shortage of Trained Professionals.
8. Can you provide examples of recent developments in the market?
September 2022: Aleva Neurotherapeutics received CE mark approval of its MRI labeling for the directSTIM deep brain stimulation (DBS) system, allowing the technology to be used in a full-body MRI environment across Europe.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Europe Neurology Monitoring Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Europe Neurology Monitoring Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Europe Neurology Monitoring Industry?
To stay informed about further developments, trends, and reports in the Europe Neurology Monitoring Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


