Key Insights
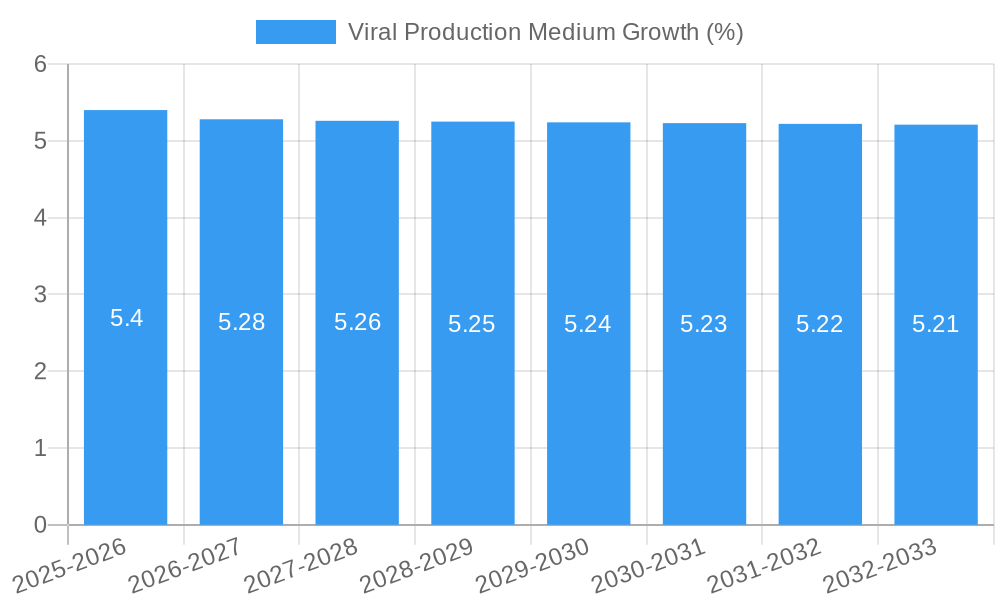
The global Viral Production Medium market is projected to experience robust growth, reaching an estimated USD 296 million in 2025 and expanding at a Compound Annual Growth Rate (CAGR) of 5.4% through 2033. This significant expansion is primarily fueled by the burgeoning demand for vaccines and biopharmaceuticals, driven by an increasing global focus on public health initiatives and the continuous development of novel therapeutic agents. The pharmaceutical segment, particularly for vaccine production, stands out as a key application, benefiting from ongoing research and development in areas like mRNA vaccines and gene therapies. Furthermore, the expanding applications of viral vectors in research, including gene editing and personalized medicine, are contributing to market dynamism. The trend towards serum-free media solutions is gaining traction due to their ability to standardize production processes, reduce batch-to-batch variability, and mitigate the risk of contamination, aligning with stringent regulatory requirements in the biopharmaceutical industry.
The market's growth trajectory is further supported by advancements in cell culture technologies and the increasing complexity of biologics requiring specialized growth environments. Key players are actively investing in R&D to develop innovative media formulations that enhance viral yields and improve the efficiency of bioprocessing. While the market is largely driven by innovation and demand, potential restraints could include the high cost associated with developing and manufacturing specialized media, as well as the complex regulatory landscape governing biopharmaceutical production. However, the persistent need for effective viral vector production for a wide range of therapeutic and diagnostic applications, coupled with a growing pipeline of biologics, is expected to outweigh these challenges, ensuring sustained market expansion across all major regions, with North America and Europe leading in adoption and innovation.
This comprehensive report offers a deep dive into the global Viral Production Medium market, meticulously analyzing its dynamics, key players, and future trajectory. Covering the historical period from 2019 to 2024, with a base year of 2025 and a forecast period extending to 2033, this study provides essential insights for industry stakeholders, researchers, and investors seeking to capitalize on this rapidly evolving sector. Discover critical data on market size, growth drivers, technological innovations, and competitive landscapes.
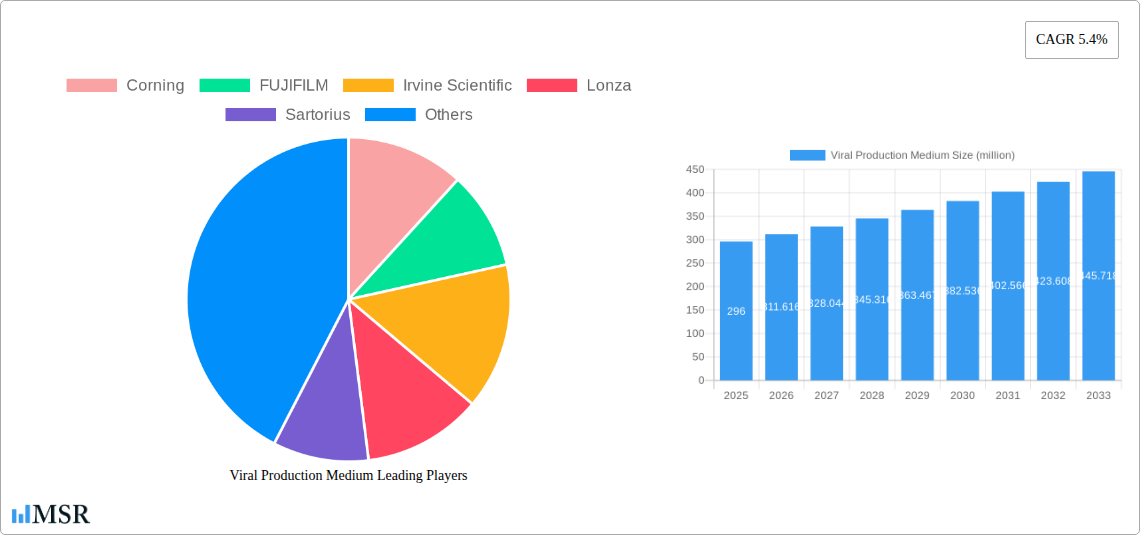
Viral Production Medium Market Concentration & Dynamics
The global Viral Production Medium market exhibits a moderate to high concentration, characterized by the presence of established multinational corporations alongside niche biotechnology firms. Innovation ecosystems are robust, driven by intense R&D efforts and collaborations aimed at enhancing viral yield, purity, and cost-effectiveness. Key players like Thermo Fisher Scientific, Lonza, and Corning command significant market share due to their extensive product portfolios and strong global distribution networks. The regulatory framework, particularly stringent in pharmaceutical applications, influences product development and market entry, necessitating compliance with GMP and other quality standards. Substitute products, such as alternative cell culture methods, pose a limited threat due to the specific requirements for viral production. End-user trends are leaning towards serum-free media formulations to reduce batch-to-batch variability and simplify downstream processing. Merger and acquisition (M&A) activities are notable, with an estimated xx M&A deal count in the past five years, as larger companies seek to acquire innovative technologies or expand their market reach. Market share distribution sees key players holding an aggregate of over 70% of the market.
Viral Production Medium Industry Insights & Trends
The Viral Production Medium market is poised for substantial growth, projected to reach over $10,000 million by 2033, exhibiting a robust Compound Annual Growth Rate (CAGR) of xx% during the forecast period (2025-2033). This expansion is primarily fueled by the escalating demand for vaccines, gene therapies, and monoclonal antibodies, all of which rely heavily on efficient viral production. Technological disruptions, particularly in the development of advanced cell culture media formulations, are a significant catalyst. Serum-free media (SFM), for instance, is witnessing accelerated adoption due to its ability to enhance viral titers, improve consistency, and reduce the risk of contamination, aligning with evolving end-user preferences for cleaner and more efficient bioprocessing. The increasing prevalence of infectious diseases and the growing need for novel therapeutics are driving the R&D pipeline, further stimulating demand for high-performance viral production media. Furthermore, significant investments in biopharmaceutical manufacturing infrastructure globally are creating a conducive environment for market expansion. The shift towards personalized medicine and the burgeoning field of viral vector-based therapies are also contributing to the upward trend. The market size in the base year of 2025 is estimated to be around $5,000 million.
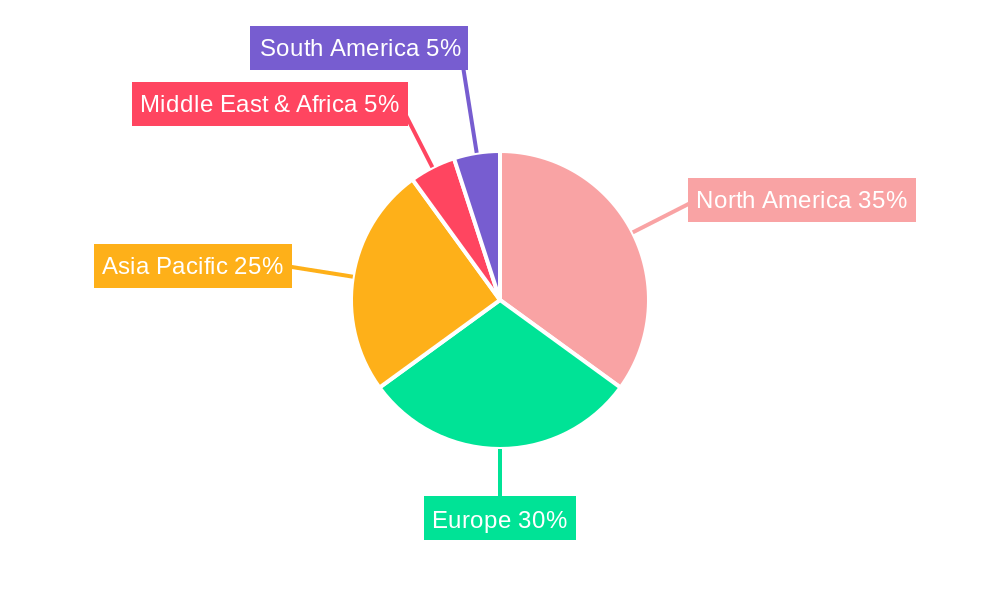
Key Markets & Segments Leading Viral Production Medium
The North America region is currently the dominant force in the global Viral Production Medium market, driven by its advanced biopharmaceutical infrastructure, substantial investments in R&D, and a high prevalence of chronic diseases necessitating novel therapeutics. Within North America, the United States spearheads market growth due to its leading position in pharmaceutical and biotechnology innovation.
Application Dominance:
- Vaccine Production: This segment is a primary growth driver, propelled by the continuous need for seasonal flu vaccines, emerging pandemic preparedness, and the development of novel prophylactic and therapeutic vaccines. The increasing global vaccination campaigns and the rapid response required for public health emergencies significantly boost demand for high-yield viral production media.
- Pharmaceutical: This broad segment encompasses the production of viral vectors for gene therapy, oncolytic viruses, and other protein-based therapeutics. The burgeoning pipeline of gene therapies and the increasing focus on biologic drug development are major contributors to this segment's growth.
- Research: Academic and commercial research institutions utilize viral production media for fundamental studies in virology, drug discovery, and the development of diagnostic tools. While smaller in market share compared to commercial production, this segment remains a crucial contributor to innovation and early-stage development.
- Others: This segment includes niche applications such as the production of viral diagnostic kits and veterinary vaccines.
Type Dominance:
- Serum-Free Medium: This type is experiencing the most rapid growth and is increasingly preferred across all applications. Its advantages, including reduced lot-to-lot variability, enhanced viral yield, and simplified purification processes, make it ideal for large-scale biopharmaceutical manufacturing. The drive towards reducing animal-derived components also contributes to its rising popularity.
- Serum-Containing Medium: While historically dominant, serum-containing media is gradually being replaced by SFM in many commercial applications. However, it remains relevant in certain research settings and for specific cell lines where serum supplementation is still considered essential for optimal cell growth and viral replication.
Viral Production Medium Product Developments
Recent product developments in the Viral Production Medium market are characterized by innovations aimed at enhancing viral yield and purity, improving process efficiency, and ensuring regulatory compliance. Companies are focusing on developing chemically defined and serum-free formulations that support robust cell growth and high-titer viral replication for applications in vaccine production and gene therapy. Advancements include media optimized for specific viral vectors, such as adeno-associated viruses (AAV) and lentiviruses, offering tailored nutrient profiles and growth factors. These technological advancements provide a competitive edge by enabling biopharmaceutical manufacturers to achieve higher productivity and reduce manufacturing costs.
Challenges in the Viral Production Medium Market
The Viral Production Medium market faces several significant challenges that impede its growth. Stringent regulatory hurdles for biopharmaceutical production require extensive validation and compliance, increasing development timelines and costs. Supply chain disruptions, particularly for critical raw materials, can impact production consistency and lead to shortages, affecting an estimated xx% of manufacturers in the past year. High manufacturing costs associated with complex media formulations and specialized production processes also present a barrier. Furthermore, intense competitive pressure among established players and the emergence of new technologies necessitate continuous innovation and cost optimization strategies, impacting profit margins.
Forces Driving Viral Production Medium Growth
Several powerful forces are propelling the Viral Production Medium market forward. Technological advancements in cell culture, including the development of advanced bioreactors and process intensification strategies, are creating a greater demand for optimized media. Economic factors, such as increased healthcare spending globally and substantial investments in the biopharmaceutical sector, are fueling market expansion. Favorable regulatory landscapes for biotherapeutics, coupled with government initiatives supporting vaccine development and infectious disease research, are creating a conducive environment for growth. The rising incidence of chronic diseases and the increasing demand for personalized medicine are also significant growth catalysts.
Challenges in the Viral Production Medium Market
Long-term growth catalysts in the Viral Production Medium market are rooted in sustained innovation and strategic market expansion. The ongoing advancements in genetic engineering and synthetic biology are paving the way for the development of next-generation viral vectors and cell therapies, creating a continuous demand for specialized and high-performance production media. Strategic partnerships between media manufacturers and biopharmaceutical companies are crucial for co-developing customized solutions that address specific therapeutic needs and optimize production processes. Furthermore, the expansion of biomanufacturing capabilities in emerging economies presents significant untapped market potential for viral production media suppliers.
Emerging Opportunities in Viral Production Medium
Emerging opportunities in the Viral Production Medium market are abundant, driven by new technologies and evolving consumer preferences. The burgeoning field of ex vivo gene therapy and the increasing use of viral vectors in cancer immunotherapy represent significant growth avenues. The development of continuous bioprocessing technologies for viral production also presents a substantial opportunity for media optimization and integration. Furthermore, the growing demand for animal-free and xeno-free media formulations for ethical and regulatory reasons, particularly in pharmaceutical applications, offers a niche but rapidly expanding market segment. The exploration of novel viral production platforms, such as insect cell cultures and plant-based systems, may also unlock new opportunities.
Leading Players in the Viral Production Medium Sector
- Corning
- FUJIFILM
- Irvine Scientific
- Lonza
- Sartorius
- STEMCELL Technologies
- Thermo Fisher Scientific
- InVitria
Key Milestones in Viral Production Medium Industry
- 2019: Introduction of novel, chemically defined serum-free media formulations for enhanced AAV production.
- 2020: Significant increase in demand for viral production media driven by the global COVID-19 pandemic, accelerating vaccine development efforts.
- 2021: Key acquisitions by major players to expand their portfolios in cell and gene therapy media.
- 2022: Advancements in single-use bioreactor technology leading to greater demand for optimized, ready-to-use media solutions.
- 2023: Increased focus on sustainability in media production, with a push for eco-friendly sourcing and packaging.
- 2024 (estimated): Launch of highly customized media for specific lentiviral vector production, demonstrating precision in formulation.
Strategic Outlook for Viral Production Medium Market
The strategic outlook for the Viral Production Medium market remains highly optimistic, driven by several key growth accelerators. The increasing pipeline of advanced therapies, including gene therapies and personalized vaccines, will continue to fuel demand for innovative and high-performance media. Strategic partnerships between media manufacturers and biopharmaceutical companies will be crucial for co-developing tailored solutions that optimize viral yield and purity. Furthermore, the ongoing trend towards automation and digitalization in biomanufacturing will create opportunities for integrated media solutions that streamline production processes. Companies that focus on developing sustainable, cost-effective, and regulatory-compliant media will be well-positioned for long-term success in this dynamic market.
Viral Production Medium Segmentation
-
1. Application
- 1.1. Vaccine Production
- 1.2. Pharmaceutical
- 1.3. Research
- 1.4. Others
-
2. Types
- 2.1. Serum-Free Medium
- 2.2. Serum-Containing Medium
Viral Production Medium Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific
Viral Production Medium REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 5.4% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Viral Production Medium Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Application
- 5.1.1. Vaccine Production
- 5.1.2. Pharmaceutical
- 5.1.3. Research
- 5.1.4. Others
- 5.2. Market Analysis, Insights and Forecast - by Types
- 5.2.1. Serum-Free Medium
- 5.2.2. Serum-Containing Medium
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Application
- 6. North America Viral Production Medium Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Application
- 6.1.1. Vaccine Production
- 6.1.2. Pharmaceutical
- 6.1.3. Research
- 6.1.4. Others
- 6.2. Market Analysis, Insights and Forecast - by Types
- 6.2.1. Serum-Free Medium
- 6.2.2. Serum-Containing Medium
- 6.1. Market Analysis, Insights and Forecast - by Application
- 7. South America Viral Production Medium Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Application
- 7.1.1. Vaccine Production
- 7.1.2. Pharmaceutical
- 7.1.3. Research
- 7.1.4. Others
- 7.2. Market Analysis, Insights and Forecast - by Types
- 7.2.1. Serum-Free Medium
- 7.2.2. Serum-Containing Medium
- 7.1. Market Analysis, Insights and Forecast - by Application
- 8. Europe Viral Production Medium Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Application
- 8.1.1. Vaccine Production
- 8.1.2. Pharmaceutical
- 8.1.3. Research
- 8.1.4. Others
- 8.2. Market Analysis, Insights and Forecast - by Types
- 8.2.1. Serum-Free Medium
- 8.2.2. Serum-Containing Medium
- 8.1. Market Analysis, Insights and Forecast - by Application
- 9. Middle East & Africa Viral Production Medium Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Application
- 9.1.1. Vaccine Production
- 9.1.2. Pharmaceutical
- 9.1.3. Research
- 9.1.4. Others
- 9.2. Market Analysis, Insights and Forecast - by Types
- 9.2.1. Serum-Free Medium
- 9.2.2. Serum-Containing Medium
- 9.1. Market Analysis, Insights and Forecast - by Application
- 10. Asia Pacific Viral Production Medium Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Application
- 10.1.1. Vaccine Production
- 10.1.2. Pharmaceutical
- 10.1.3. Research
- 10.1.4. Others
- 10.2. Market Analysis, Insights and Forecast - by Types
- 10.2.1. Serum-Free Medium
- 10.2.2. Serum-Containing Medium
- 10.1. Market Analysis, Insights and Forecast - by Application
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 Corning
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 FUJIFILM
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Irvine Scientific
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Lonza
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Sartorius
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 STEMCELL Technologies
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Thermo Fisher Scientific
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 InVitria
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.1 Corning
List of Figures
- Figure 1: Global Viral Production Medium Revenue Breakdown (million, %) by Region 2024 & 2032
- Figure 2: North America Viral Production Medium Revenue (million), by Application 2024 & 2032
- Figure 3: North America Viral Production Medium Revenue Share (%), by Application 2024 & 2032
- Figure 4: North America Viral Production Medium Revenue (million), by Types 2024 & 2032
- Figure 5: North America Viral Production Medium Revenue Share (%), by Types 2024 & 2032
- Figure 6: North America Viral Production Medium Revenue (million), by Country 2024 & 2032
- Figure 7: North America Viral Production Medium Revenue Share (%), by Country 2024 & 2032
- Figure 8: South America Viral Production Medium Revenue (million), by Application 2024 & 2032
- Figure 9: South America Viral Production Medium Revenue Share (%), by Application 2024 & 2032
- Figure 10: South America Viral Production Medium Revenue (million), by Types 2024 & 2032
- Figure 11: South America Viral Production Medium Revenue Share (%), by Types 2024 & 2032
- Figure 12: South America Viral Production Medium Revenue (million), by Country 2024 & 2032
- Figure 13: South America Viral Production Medium Revenue Share (%), by Country 2024 & 2032
- Figure 14: Europe Viral Production Medium Revenue (million), by Application 2024 & 2032
- Figure 15: Europe Viral Production Medium Revenue Share (%), by Application 2024 & 2032
- Figure 16: Europe Viral Production Medium Revenue (million), by Types 2024 & 2032
- Figure 17: Europe Viral Production Medium Revenue Share (%), by Types 2024 & 2032
- Figure 18: Europe Viral Production Medium Revenue (million), by Country 2024 & 2032
- Figure 19: Europe Viral Production Medium Revenue Share (%), by Country 2024 & 2032
- Figure 20: Middle East & Africa Viral Production Medium Revenue (million), by Application 2024 & 2032
- Figure 21: Middle East & Africa Viral Production Medium Revenue Share (%), by Application 2024 & 2032
- Figure 22: Middle East & Africa Viral Production Medium Revenue (million), by Types 2024 & 2032
- Figure 23: Middle East & Africa Viral Production Medium Revenue Share (%), by Types 2024 & 2032
- Figure 24: Middle East & Africa Viral Production Medium Revenue (million), by Country 2024 & 2032
- Figure 25: Middle East & Africa Viral Production Medium Revenue Share (%), by Country 2024 & 2032
- Figure 26: Asia Pacific Viral Production Medium Revenue (million), by Application 2024 & 2032
- Figure 27: Asia Pacific Viral Production Medium Revenue Share (%), by Application 2024 & 2032
- Figure 28: Asia Pacific Viral Production Medium Revenue (million), by Types 2024 & 2032
- Figure 29: Asia Pacific Viral Production Medium Revenue Share (%), by Types 2024 & 2032
- Figure 30: Asia Pacific Viral Production Medium Revenue (million), by Country 2024 & 2032
- Figure 31: Asia Pacific Viral Production Medium Revenue Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global Viral Production Medium Revenue million Forecast, by Region 2019 & 2032
- Table 2: Global Viral Production Medium Revenue million Forecast, by Application 2019 & 2032
- Table 3: Global Viral Production Medium Revenue million Forecast, by Types 2019 & 2032
- Table 4: Global Viral Production Medium Revenue million Forecast, by Region 2019 & 2032
- Table 5: Global Viral Production Medium Revenue million Forecast, by Application 2019 & 2032
- Table 6: Global Viral Production Medium Revenue million Forecast, by Types 2019 & 2032
- Table 7: Global Viral Production Medium Revenue million Forecast, by Country 2019 & 2032
- Table 8: United States Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 9: Canada Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 10: Mexico Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 11: Global Viral Production Medium Revenue million Forecast, by Application 2019 & 2032
- Table 12: Global Viral Production Medium Revenue million Forecast, by Types 2019 & 2032
- Table 13: Global Viral Production Medium Revenue million Forecast, by Country 2019 & 2032
- Table 14: Brazil Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 15: Argentina Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 16: Rest of South America Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 17: Global Viral Production Medium Revenue million Forecast, by Application 2019 & 2032
- Table 18: Global Viral Production Medium Revenue million Forecast, by Types 2019 & 2032
- Table 19: Global Viral Production Medium Revenue million Forecast, by Country 2019 & 2032
- Table 20: United Kingdom Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 21: Germany Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 22: France Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 23: Italy Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 24: Spain Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 25: Russia Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 26: Benelux Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 27: Nordics Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 28: Rest of Europe Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 29: Global Viral Production Medium Revenue million Forecast, by Application 2019 & 2032
- Table 30: Global Viral Production Medium Revenue million Forecast, by Types 2019 & 2032
- Table 31: Global Viral Production Medium Revenue million Forecast, by Country 2019 & 2032
- Table 32: Turkey Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 33: Israel Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 34: GCC Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 35: North Africa Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 36: South Africa Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 37: Rest of Middle East & Africa Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 38: Global Viral Production Medium Revenue million Forecast, by Application 2019 & 2032
- Table 39: Global Viral Production Medium Revenue million Forecast, by Types 2019 & 2032
- Table 40: Global Viral Production Medium Revenue million Forecast, by Country 2019 & 2032
- Table 41: China Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 42: India Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 43: Japan Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 44: South Korea Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 45: ASEAN Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 46: Oceania Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
- Table 47: Rest of Asia Pacific Viral Production Medium Revenue (million) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Viral Production Medium?
The projected CAGR is approximately 5.4%.
2. Which companies are prominent players in the Viral Production Medium?
Key companies in the market include Corning, FUJIFILM, Irvine Scientific, Lonza, Sartorius, STEMCELL Technologies, Thermo Fisher Scientific, InVitria.
3. What are the main segments of the Viral Production Medium?
The market segments include Application, Types.
4. Can you provide details about the market size?
The market size is estimated to be USD 296 million as of 2022.
5. What are some drivers contributing to market growth?
N/A
6. What are the notable trends driving market growth?
N/A
7. Are there any restraints impacting market growth?
N/A
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 2900.00, USD 4350.00, and USD 5800.00 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Viral Production Medium," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Viral Production Medium report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Viral Production Medium?
To stay informed about further developments, trends, and reports in the Viral Production Medium, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


