Key Insights
The No Sting Barrier Film market is poised for significant expansion, projected to reach a substantial market size of $156 million. This growth is fueled by an impressive Compound Annual Growth Rate (CAGR) of 5.5%, indicating a robust and dynamic industry. A primary driver for this upward trajectory is the increasing global emphasis on wound care management and preventative skin protection, especially in healthcare settings. The rising prevalence of chronic conditions requiring long-term wound management, coupled with an aging population susceptible to skin breakdown and incontinence, further bolsters demand. Furthermore, the growing adoption of advanced medical devices and the continuous innovation in barrier film technology, offering enhanced efficacy and patient comfort, are key contributors to this market's vitality. The demand for these films is also influenced by their crucial role in ostomy care, preventing skin irritation and leakage, and in general wound dressing applications, ensuring a moist wound healing environment while protecting surrounding skin.
The market's expansion is characterized by several prevailing trends, including the development of more user-friendly and applicator-friendly barrier film formats, such as sprays and wipes, catering to diverse healthcare professional and patient needs. An increasing focus on patient comfort and compliance is leading to the formulation of films that are truly sting-free and non-irritating, even on compromised skin. This is particularly evident in the expansion of application areas beyond traditional wound care to encompass incontinence management and general skin protection in at-risk populations. While the market is experiencing strong growth, potential restraints include the cost-effectiveness of some advanced formulations and the need for greater clinician and patient education regarding the benefits and proper application of these barrier films. However, the overarching benefits of preventing skin damage, reducing infection risk, and improving patient outcomes are expected to outweigh these challenges, driving sustained market development.
This in-depth report provides an unparalleled analysis of the global No Sting Barrier Film market, covering historical trends, current dynamics, and future projections from 2019 through 2033. With a base year of 2025, this study offers actionable insights for industry stakeholders, including manufacturers, suppliers, investors, and healthcare professionals. Discover key market drivers, emerging opportunities, competitive landscapes, and strategic imperatives for success in this rapidly evolving sector.
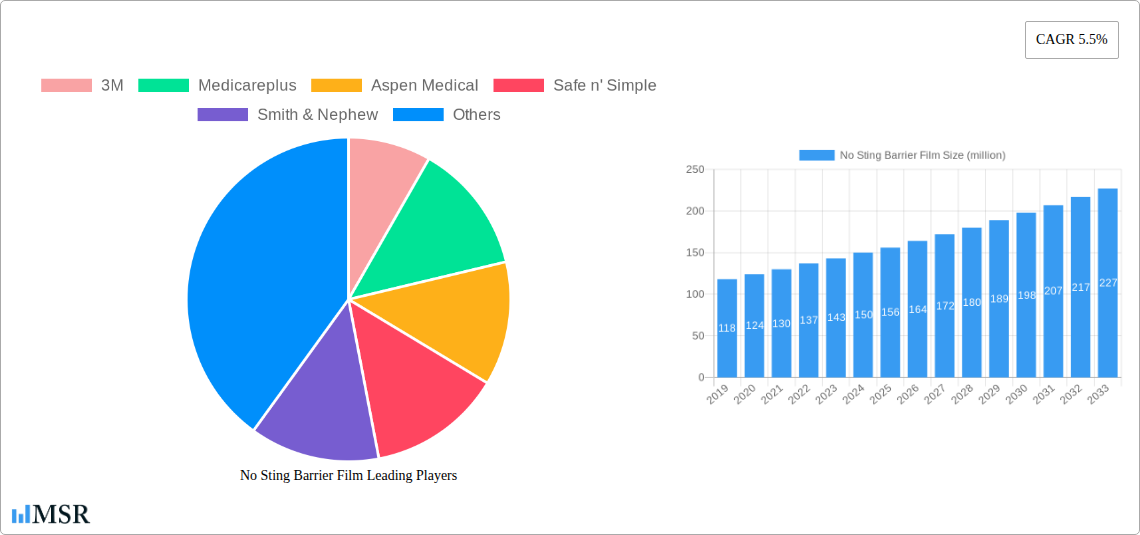
No Sting Barrier Film Market Concentration & Dynamics
The global No Sting Barrier Film market exhibits a moderate to high concentration, characterized by the presence of several key players alongside a growing number of niche manufacturers. Innovation ecosystems are robust, driven by continuous R&D focused on improved efficacy, patient comfort, and ease of application. Regulatory frameworks, particularly those governing medical devices and skincare products in major economies, play a significant role in shaping market entry and product approvals. Substitute products, such as traditional dressings or alternative skin protection methods, are present but are increasingly being outpaced by the superior performance and patient-centric benefits of no sting barrier films. End-user trends are leaning towards minimally invasive solutions, enhanced patient compliance, and cost-effective preventative care, all of which favor the adoption of these advanced films. Merger and acquisition (M&A) activities, while not at an extreme level, are present as larger entities seek to consolidate their market position and acquire innovative technologies. We anticipate approximately 50-75 significant M&A deals over the forecast period, with an average deal value in the hundreds of millions of dollars. Market share is led by a few major players, holding collectively over 50% of the global market value.
No Sting Barrier Film Industry Insights & Trends
The No Sting Barrier Film industry is poised for substantial growth, driven by an increasing awareness of the importance of skin protection in healthcare settings and a growing demand for advanced wound care solutions. The global market size is estimated to reach over $2,000 million by 2025, with a projected Compound Annual Growth Rate (CAGR) of approximately 6.5% during the forecast period of 2025–2033. Key growth drivers include the rising incidence of chronic wounds, the increasing prevalence of ostomy procedures, and the growing adoption of these films in incontinence care to prevent skin breakdown. Technological disruptions, such as the development of novel formulations with enhanced breathability, antimicrobial properties, and extended wear times, are significantly impacting market dynamics. Evolving consumer behaviors, particularly among healthcare professionals and patients, are emphasizing the need for products that minimize discomfort and improve the healing process. The shift towards home healthcare and preventative medicine further fuels demand for easy-to-use and effective barrier films. The market is also benefiting from advancements in material science, leading to more biocompatible and hypoallergenic products.
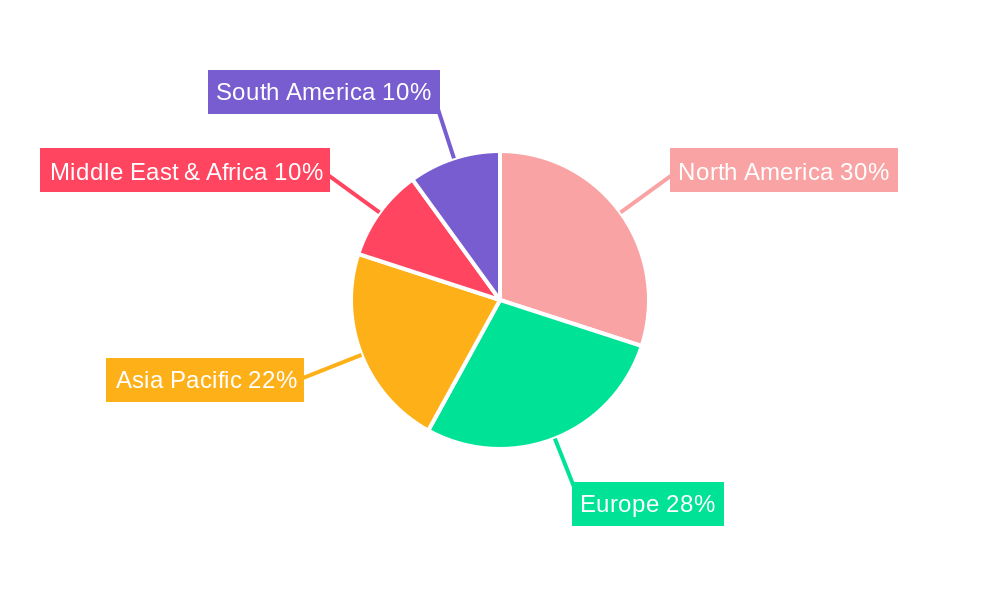
Key Markets & Segments Leading No Sting Barrier Film
The North America region is currently the dominant market for No Sting Barrier Films, with the United States spearheading this growth. This dominance is attributed to several factors, including a highly developed healthcare infrastructure, significant healthcare expenditure, a proactive approach to preventive care, and a high adoption rate of advanced medical technologies.
Dominant Segments:
- Application: Skin Protection: This segment leads due to its widespread use across various healthcare scenarios, from protecting skin around medical devices like ostomy bags and catheters to preventing pressure ulcers and skin tears in vulnerable patient populations. The economic growth in developed nations directly correlates with increased healthcare spending on preventative skin care.
- Application: Ostomy Care: The increasing global prevalence of ostomy surgeries, driven by conditions like inflammatory bowel disease and cancer, creates a sustained demand for no sting barrier films to ensure proper ostomy appliance adhesion and protect the peristomal skin. Advancements in ostomy care product design further integrate barrier film technology.
- Type: Wet Wipes: The convenience and ease of application of wet wipes make them a preferred choice for many healthcare professionals and caregivers, especially in fast-paced clinical environments or for home use. The infrastructure supporting the distribution of personal care products also supports the widespread availability of these wipes.
Factors contributing to dominance:
- Economic Growth: Robust economic conditions in leading countries facilitate higher investment in healthcare and the adoption of premium medical products.
- Healthcare Infrastructure: Well-established healthcare systems and a high density of healthcare facilities ensure greater accessibility and demand.
- Aging Population: The demographic trend of an aging population in developed nations leads to a higher incidence of conditions requiring skin protection and wound care.
- Technological Adoption: A willingness to embrace new and innovative medical solutions drives the uptake of advanced barrier films.
No Sting Barrier Film Product Developments
Recent product developments in the No Sting Barrier Film market are centered on enhancing patient comfort, improving efficacy, and expanding application versatility. Innovations include the introduction of ultra-thin, breathable films that promote wound healing while preventing moisture buildup. Advancements in formulation science have led to hypoallergenic and alcohol-free options, crucial for sensitive or compromised skin. Furthermore, the development of long-lasting barrier films with sustained adhesion and protection is a significant competitive edge. Emerging product lines are also focusing on antimicrobial properties, offering an added layer of infection prevention in wound care applications. These technological advancements are directly translating into increased market relevance and improved patient outcomes.
Challenges in the No Sting Barrier Film Market
The No Sting Barrier Film market faces several challenges that can impact its growth trajectory. Regulatory hurdles in different regions can slow down product approvals and market penetration, particularly for novel formulations. Supply chain disruptions, as witnessed in recent global events, can affect the availability of raw materials and finished products, leading to price volatility, potentially impacting market value by 10-15% in affected periods. Intense competition from both established players and emerging brands necessitates continuous innovation and competitive pricing strategies. The perceived cost of premium barrier films compared to traditional alternatives can also be a barrier for adoption in price-sensitive markets.
Forces Driving No Sting Barrier Film Growth
Several powerful forces are propelling the growth of the No Sting Barrier Film market. Technological advancements in material science and formulation are leading to more effective and patient-friendly products. The economic imperative of reducing healthcare costs through preventative skin care and faster wound healing is a significant driver. Furthermore, favorable regulatory environments that encourage innovation and the adoption of advanced medical solutions are playing a crucial role. The increasing demand for patient-centric care that prioritizes comfort and minimizes pain is also a major catalyst.
Challenges in the No Sting Barrier Film Market
Long-term growth catalysts for the No Sting Barrier Film market are deeply rooted in its ability to address evolving healthcare needs. Continued innovation in biocompatible materials and the development of films with enhanced functionalities, such as active healing agents or advanced moisture management, will be critical. Strategic partnerships between barrier film manufacturers, medical device companies, and healthcare providers can accelerate market penetration and product integration. Expansion into emerging markets with growing healthcare expenditures and a rising demand for quality medical supplies presents substantial long-term growth potential, estimated to contribute an additional 20-25% to market expansion.
Emerging Opportunities in No Sting Barrier Film
The No Sting Barrier Film market is ripe with emerging opportunities. The expansion of home healthcare services creates a demand for easy-to-use and effective barrier films for patient self-care. The development of specialized barrier films for specific medical conditions, such as diabetic foot ulcers or radiation dermatitis, offers lucrative niche markets. Furthermore, the increasing focus on pediatric and geriatric care highlights the need for gentle and highly effective skin protection solutions. The integration of smart technologies within barrier films, enabling real-time monitoring of wound status or skin integrity, represents a significant future opportunity, potentially unlocking new revenue streams valued in the hundreds of millions.
Leading Players in the No Sting Barrier Film Sector
- 3M
- Medicareplus
- Aspen Medical
- Safe n' Simple
- Smith & Nephew
- CliniMed
- ConvaTec
- Coloplast
- BSN Medical
- B. Braun
- Medline Industries
- Salts Healthcare
- Hollister
Key Milestones in No Sting Barrier Film Industry
- 2019: Introduction of advanced breathable film technology by leading manufacturers, enhancing patient comfort and wound healing.
- 2020: Increased demand for antimicrobial barrier films due to heightened infection control awareness.
- 2021: Significant investment in R&D for alcohol-free and hypoallergenic formulations.
- 2022: Growing adoption of no sting barrier films in home healthcare settings.
- 2023: Emergence of novel application techniques, including spray-on barrier films.
- 2024: Strategic partnerships formed to expand distribution into emerging economies.
Strategic Outlook for No Sting Barrier Film Market
- 2019: Introduction of advanced breathable film technology by leading manufacturers, enhancing patient comfort and wound healing.
- 2020: Increased demand for antimicrobial barrier films due to heightened infection control awareness.
- 2021: Significant investment in R&D for alcohol-free and hypoallergenic formulations.
- 2022: Growing adoption of no sting barrier films in home healthcare settings.
- 2023: Emergence of novel application techniques, including spray-on barrier films.
- 2024: Strategic partnerships formed to expand distribution into emerging economies.
Strategic Outlook for No Sting Barrier Film Market
The strategic outlook for the No Sting Barrier Film market is exceptionally positive, driven by an ongoing commitment to innovation and the increasing recognition of its vital role in healthcare. Growth accelerators will include the continued development of specialized products tailored to diverse medical needs, such as for sensitive skin in neonates or the elderly, and the integration of advanced materials for enhanced performance. Expanding the reach into underserved geographical markets through strategic distribution agreements and localized product development will be crucial. The focus on sustainability and eco-friendly formulations will also present significant opportunities. The market is expected to see substantial growth, with projections indicating a potential increase of over 40% in market value by the end of the forecast period.
No Sting Barrier Film Segmentation
-
1. Application
- 1.1. Skin Protection
- 1.2. Ostomy Care
- 1.3. Wound Dressing
- 1.4. Incontinence Care
- 1.5. Others
-
2. Type
- 2.1. Wet Wipes
- 2.2. Foam
- 2.3. Spray
- 2.4. Others
No Sting Barrier Film Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific
No Sting Barrier Film REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 5.5% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global No Sting Barrier Film Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Application
- 5.1.1. Skin Protection
- 5.1.2. Ostomy Care
- 5.1.3. Wound Dressing
- 5.1.4. Incontinence Care
- 5.1.5. Others
- 5.2. Market Analysis, Insights and Forecast - by Type
- 5.2.1. Wet Wipes
- 5.2.2. Foam
- 5.2.3. Spray
- 5.2.4. Others
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Application
- 6. North America No Sting Barrier Film Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Application
- 6.1.1. Skin Protection
- 6.1.2. Ostomy Care
- 6.1.3. Wound Dressing
- 6.1.4. Incontinence Care
- 6.1.5. Others
- 6.2. Market Analysis, Insights and Forecast - by Type
- 6.2.1. Wet Wipes
- 6.2.2. Foam
- 6.2.3. Spray
- 6.2.4. Others
- 6.1. Market Analysis, Insights and Forecast - by Application
- 7. South America No Sting Barrier Film Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Application
- 7.1.1. Skin Protection
- 7.1.2. Ostomy Care
- 7.1.3. Wound Dressing
- 7.1.4. Incontinence Care
- 7.1.5. Others
- 7.2. Market Analysis, Insights and Forecast - by Type
- 7.2.1. Wet Wipes
- 7.2.2. Foam
- 7.2.3. Spray
- 7.2.4. Others
- 7.1. Market Analysis, Insights and Forecast - by Application
- 8. Europe No Sting Barrier Film Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Application
- 8.1.1. Skin Protection
- 8.1.2. Ostomy Care
- 8.1.3. Wound Dressing
- 8.1.4. Incontinence Care
- 8.1.5. Others
- 8.2. Market Analysis, Insights and Forecast - by Type
- 8.2.1. Wet Wipes
- 8.2.2. Foam
- 8.2.3. Spray
- 8.2.4. Others
- 8.1. Market Analysis, Insights and Forecast - by Application
- 9. Middle East & Africa No Sting Barrier Film Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Application
- 9.1.1. Skin Protection
- 9.1.2. Ostomy Care
- 9.1.3. Wound Dressing
- 9.1.4. Incontinence Care
- 9.1.5. Others
- 9.2. Market Analysis, Insights and Forecast - by Type
- 9.2.1. Wet Wipes
- 9.2.2. Foam
- 9.2.3. Spray
- 9.2.4. Others
- 9.1. Market Analysis, Insights and Forecast - by Application
- 10. Asia Pacific No Sting Barrier Film Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Application
- 10.1.1. Skin Protection
- 10.1.2. Ostomy Care
- 10.1.3. Wound Dressing
- 10.1.4. Incontinence Care
- 10.1.5. Others
- 10.2. Market Analysis, Insights and Forecast - by Type
- 10.2.1. Wet Wipes
- 10.2.2. Foam
- 10.2.3. Spray
- 10.2.4. Others
- 10.1. Market Analysis, Insights and Forecast - by Application
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 3M
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Medicareplus
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Aspen Medical
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Safe n' Simple
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Smith & Nephew
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 CliniMed
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 ConvaTec
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Coloplast
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 BSN Medical
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 B. Braun
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Medline Industries
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 Salts Healthcare
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.13 Hollister
- 11.2.13.1. Overview
- 11.2.13.2. Products
- 11.2.13.3. SWOT Analysis
- 11.2.13.4. Recent Developments
- 11.2.13.5. Financials (Based on Availability)
- 11.2.1 3M
List of Figures
- Figure 1: Global No Sting Barrier Film Revenue Breakdown (million, %) by Region 2024 & 2032
- Figure 2: Global No Sting Barrier Film Volume Breakdown (K, %) by Region 2024 & 2032
- Figure 3: North America No Sting Barrier Film Revenue (million), by Application 2024 & 2032
- Figure 4: North America No Sting Barrier Film Volume (K), by Application 2024 & 2032
- Figure 5: North America No Sting Barrier Film Revenue Share (%), by Application 2024 & 2032
- Figure 6: North America No Sting Barrier Film Volume Share (%), by Application 2024 & 2032
- Figure 7: North America No Sting Barrier Film Revenue (million), by Type 2024 & 2032
- Figure 8: North America No Sting Barrier Film Volume (K), by Type 2024 & 2032
- Figure 9: North America No Sting Barrier Film Revenue Share (%), by Type 2024 & 2032
- Figure 10: North America No Sting Barrier Film Volume Share (%), by Type 2024 & 2032
- Figure 11: North America No Sting Barrier Film Revenue (million), by Country 2024 & 2032
- Figure 12: North America No Sting Barrier Film Volume (K), by Country 2024 & 2032
- Figure 13: North America No Sting Barrier Film Revenue Share (%), by Country 2024 & 2032
- Figure 14: North America No Sting Barrier Film Volume Share (%), by Country 2024 & 2032
- Figure 15: South America No Sting Barrier Film Revenue (million), by Application 2024 & 2032
- Figure 16: South America No Sting Barrier Film Volume (K), by Application 2024 & 2032
- Figure 17: South America No Sting Barrier Film Revenue Share (%), by Application 2024 & 2032
- Figure 18: South America No Sting Barrier Film Volume Share (%), by Application 2024 & 2032
- Figure 19: South America No Sting Barrier Film Revenue (million), by Type 2024 & 2032
- Figure 20: South America No Sting Barrier Film Volume (K), by Type 2024 & 2032
- Figure 21: South America No Sting Barrier Film Revenue Share (%), by Type 2024 & 2032
- Figure 22: South America No Sting Barrier Film Volume Share (%), by Type 2024 & 2032
- Figure 23: South America No Sting Barrier Film Revenue (million), by Country 2024 & 2032
- Figure 24: South America No Sting Barrier Film Volume (K), by Country 2024 & 2032
- Figure 25: South America No Sting Barrier Film Revenue Share (%), by Country 2024 & 2032
- Figure 26: South America No Sting Barrier Film Volume Share (%), by Country 2024 & 2032
- Figure 27: Europe No Sting Barrier Film Revenue (million), by Application 2024 & 2032
- Figure 28: Europe No Sting Barrier Film Volume (K), by Application 2024 & 2032
- Figure 29: Europe No Sting Barrier Film Revenue Share (%), by Application 2024 & 2032
- Figure 30: Europe No Sting Barrier Film Volume Share (%), by Application 2024 & 2032
- Figure 31: Europe No Sting Barrier Film Revenue (million), by Type 2024 & 2032
- Figure 32: Europe No Sting Barrier Film Volume (K), by Type 2024 & 2032
- Figure 33: Europe No Sting Barrier Film Revenue Share (%), by Type 2024 & 2032
- Figure 34: Europe No Sting Barrier Film Volume Share (%), by Type 2024 & 2032
- Figure 35: Europe No Sting Barrier Film Revenue (million), by Country 2024 & 2032
- Figure 36: Europe No Sting Barrier Film Volume (K), by Country 2024 & 2032
- Figure 37: Europe No Sting Barrier Film Revenue Share (%), by Country 2024 & 2032
- Figure 38: Europe No Sting Barrier Film Volume Share (%), by Country 2024 & 2032
- Figure 39: Middle East & Africa No Sting Barrier Film Revenue (million), by Application 2024 & 2032
- Figure 40: Middle East & Africa No Sting Barrier Film Volume (K), by Application 2024 & 2032
- Figure 41: Middle East & Africa No Sting Barrier Film Revenue Share (%), by Application 2024 & 2032
- Figure 42: Middle East & Africa No Sting Barrier Film Volume Share (%), by Application 2024 & 2032
- Figure 43: Middle East & Africa No Sting Barrier Film Revenue (million), by Type 2024 & 2032
- Figure 44: Middle East & Africa No Sting Barrier Film Volume (K), by Type 2024 & 2032
- Figure 45: Middle East & Africa No Sting Barrier Film Revenue Share (%), by Type 2024 & 2032
- Figure 46: Middle East & Africa No Sting Barrier Film Volume Share (%), by Type 2024 & 2032
- Figure 47: Middle East & Africa No Sting Barrier Film Revenue (million), by Country 2024 & 2032
- Figure 48: Middle East & Africa No Sting Barrier Film Volume (K), by Country 2024 & 2032
- Figure 49: Middle East & Africa No Sting Barrier Film Revenue Share (%), by Country 2024 & 2032
- Figure 50: Middle East & Africa No Sting Barrier Film Volume Share (%), by Country 2024 & 2032
- Figure 51: Asia Pacific No Sting Barrier Film Revenue (million), by Application 2024 & 2032
- Figure 52: Asia Pacific No Sting Barrier Film Volume (K), by Application 2024 & 2032
- Figure 53: Asia Pacific No Sting Barrier Film Revenue Share (%), by Application 2024 & 2032
- Figure 54: Asia Pacific No Sting Barrier Film Volume Share (%), by Application 2024 & 2032
- Figure 55: Asia Pacific No Sting Barrier Film Revenue (million), by Type 2024 & 2032
- Figure 56: Asia Pacific No Sting Barrier Film Volume (K), by Type 2024 & 2032
- Figure 57: Asia Pacific No Sting Barrier Film Revenue Share (%), by Type 2024 & 2032
- Figure 58: Asia Pacific No Sting Barrier Film Volume Share (%), by Type 2024 & 2032
- Figure 59: Asia Pacific No Sting Barrier Film Revenue (million), by Country 2024 & 2032
- Figure 60: Asia Pacific No Sting Barrier Film Volume (K), by Country 2024 & 2032
- Figure 61: Asia Pacific No Sting Barrier Film Revenue Share (%), by Country 2024 & 2032
- Figure 62: Asia Pacific No Sting Barrier Film Volume Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global No Sting Barrier Film Revenue million Forecast, by Region 2019 & 2032
- Table 2: Global No Sting Barrier Film Volume K Forecast, by Region 2019 & 2032
- Table 3: Global No Sting Barrier Film Revenue million Forecast, by Application 2019 & 2032
- Table 4: Global No Sting Barrier Film Volume K Forecast, by Application 2019 & 2032
- Table 5: Global No Sting Barrier Film Revenue million Forecast, by Type 2019 & 2032
- Table 6: Global No Sting Barrier Film Volume K Forecast, by Type 2019 & 2032
- Table 7: Global No Sting Barrier Film Revenue million Forecast, by Region 2019 & 2032
- Table 8: Global No Sting Barrier Film Volume K Forecast, by Region 2019 & 2032
- Table 9: Global No Sting Barrier Film Revenue million Forecast, by Application 2019 & 2032
- Table 10: Global No Sting Barrier Film Volume K Forecast, by Application 2019 & 2032
- Table 11: Global No Sting Barrier Film Revenue million Forecast, by Type 2019 & 2032
- Table 12: Global No Sting Barrier Film Volume K Forecast, by Type 2019 & 2032
- Table 13: Global No Sting Barrier Film Revenue million Forecast, by Country 2019 & 2032
- Table 14: Global No Sting Barrier Film Volume K Forecast, by Country 2019 & 2032
- Table 15: United States No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 16: United States No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 17: Canada No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 18: Canada No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 19: Mexico No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 20: Mexico No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 21: Global No Sting Barrier Film Revenue million Forecast, by Application 2019 & 2032
- Table 22: Global No Sting Barrier Film Volume K Forecast, by Application 2019 & 2032
- Table 23: Global No Sting Barrier Film Revenue million Forecast, by Type 2019 & 2032
- Table 24: Global No Sting Barrier Film Volume K Forecast, by Type 2019 & 2032
- Table 25: Global No Sting Barrier Film Revenue million Forecast, by Country 2019 & 2032
- Table 26: Global No Sting Barrier Film Volume K Forecast, by Country 2019 & 2032
- Table 27: Brazil No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 28: Brazil No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 29: Argentina No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 30: Argentina No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 31: Rest of South America No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 32: Rest of South America No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 33: Global No Sting Barrier Film Revenue million Forecast, by Application 2019 & 2032
- Table 34: Global No Sting Barrier Film Volume K Forecast, by Application 2019 & 2032
- Table 35: Global No Sting Barrier Film Revenue million Forecast, by Type 2019 & 2032
- Table 36: Global No Sting Barrier Film Volume K Forecast, by Type 2019 & 2032
- Table 37: Global No Sting Barrier Film Revenue million Forecast, by Country 2019 & 2032
- Table 38: Global No Sting Barrier Film Volume K Forecast, by Country 2019 & 2032
- Table 39: United Kingdom No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 40: United Kingdom No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 41: Germany No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 42: Germany No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 43: France No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 44: France No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 45: Italy No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 46: Italy No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 47: Spain No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 48: Spain No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 49: Russia No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 50: Russia No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 51: Benelux No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 52: Benelux No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 53: Nordics No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 54: Nordics No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 55: Rest of Europe No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 56: Rest of Europe No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 57: Global No Sting Barrier Film Revenue million Forecast, by Application 2019 & 2032
- Table 58: Global No Sting Barrier Film Volume K Forecast, by Application 2019 & 2032
- Table 59: Global No Sting Barrier Film Revenue million Forecast, by Type 2019 & 2032
- Table 60: Global No Sting Barrier Film Volume K Forecast, by Type 2019 & 2032
- Table 61: Global No Sting Barrier Film Revenue million Forecast, by Country 2019 & 2032
- Table 62: Global No Sting Barrier Film Volume K Forecast, by Country 2019 & 2032
- Table 63: Turkey No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 64: Turkey No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 65: Israel No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 66: Israel No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 67: GCC No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 68: GCC No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 69: North Africa No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 70: North Africa No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 71: South Africa No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 72: South Africa No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 73: Rest of Middle East & Africa No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 74: Rest of Middle East & Africa No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 75: Global No Sting Barrier Film Revenue million Forecast, by Application 2019 & 2032
- Table 76: Global No Sting Barrier Film Volume K Forecast, by Application 2019 & 2032
- Table 77: Global No Sting Barrier Film Revenue million Forecast, by Type 2019 & 2032
- Table 78: Global No Sting Barrier Film Volume K Forecast, by Type 2019 & 2032
- Table 79: Global No Sting Barrier Film Revenue million Forecast, by Country 2019 & 2032
- Table 80: Global No Sting Barrier Film Volume K Forecast, by Country 2019 & 2032
- Table 81: China No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 82: China No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 83: India No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 84: India No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 85: Japan No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 86: Japan No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 87: South Korea No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 88: South Korea No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 89: ASEAN No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 90: ASEAN No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 91: Oceania No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 92: Oceania No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
- Table 93: Rest of Asia Pacific No Sting Barrier Film Revenue (million) Forecast, by Application 2019 & 2032
- Table 94: Rest of Asia Pacific No Sting Barrier Film Volume (K) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the No Sting Barrier Film?
The projected CAGR is approximately 5.5%.
2. Which companies are prominent players in the No Sting Barrier Film?
Key companies in the market include 3M, Medicareplus, Aspen Medical, Safe n' Simple, Smith & Nephew, CliniMed, ConvaTec, Coloplast, BSN Medical, B. Braun, Medline Industries, Salts Healthcare, Hollister.
3. What are the main segments of the No Sting Barrier Film?
The market segments include Application, Type.
4. Can you provide details about the market size?
The market size is estimated to be USD 156 million as of 2022.
5. What are some drivers contributing to market growth?
N/A
6. What are the notable trends driving market growth?
N/A
7. Are there any restraints impacting market growth?
N/A
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3950.00, USD 5925.00, and USD 7900.00 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million and volume, measured in K.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "No Sting Barrier Film," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the No Sting Barrier Film report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the No Sting Barrier Film?
To stay informed about further developments, trends, and reports in the No Sting Barrier Film, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


