Key Insights
The Acute Lymphoblastic Leukemia (ALL) market is poised for significant expansion, driven by advancements in treatment modalities and increasing global incidence of the disease. The market was valued at $2270.5 million in 2024 and is projected to experience a robust Compound Annual Growth Rate (CAGR) of 5.61% through 2033. This growth is primarily fueled by innovations in targeted therapies and immunotherapy, which offer more personalized and effective treatment options compared to traditional chemotherapy alone. The increasing sophistication of stem cell transplantation techniques also contributes to improved patient outcomes and market growth. Furthermore, a growing awareness of early diagnosis and access to advanced healthcare infrastructure in emerging economies are expected to further accelerate market penetration.
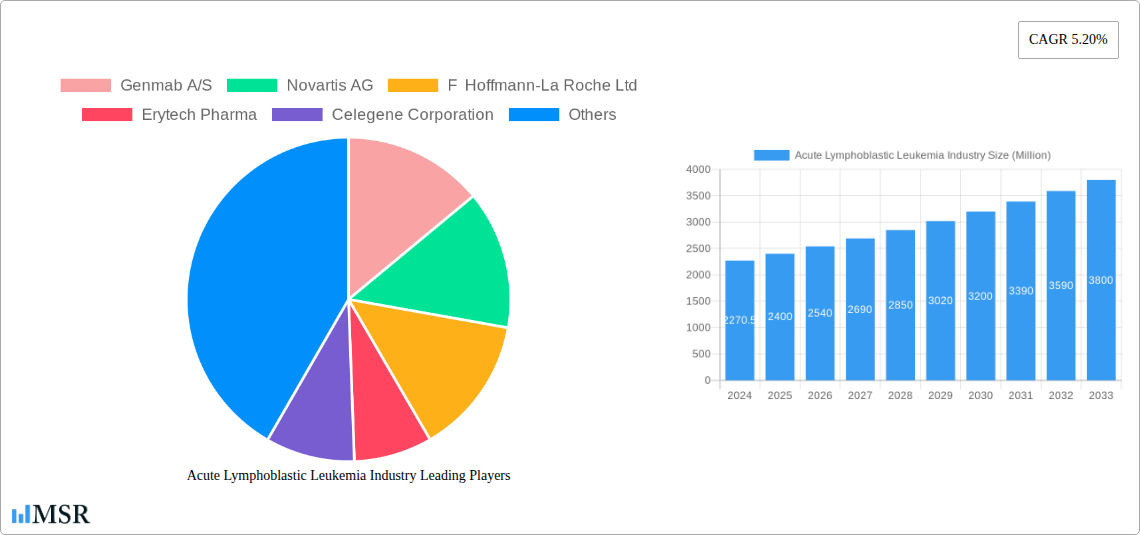
Acute Lymphoblastic Leukemia Industry Market Size (In Billion)

The ALL market is segmented by cell type, including B-cell ALL and T-cell ALL, with Philadel (Philadelphia chromosome-positive ALL) representing a distinct subgroup. Treatment strategies are diverse, encompassing chemotherapy regimens like Hyper-CVAD, CALGB 8811, and Linker, alongside nucleoside inhibitors and other novel chemotherapeutic agents. Targeted therapy and radiation therapy also play crucial roles, with stem cell transplantation offering a definitive solution for many. Key players such as Novartis AG, F. Hoffmann-La Roche Ltd, Bristol Myers Squibb Company, and Pfizer Inc. are heavily invested in research and development, launching innovative drugs and therapies that address unmet medical needs. Geographically, North America currently dominates the market, followed by Europe, with the Asia Pacific region demonstrating substantial growth potential due to rising healthcare expenditure and an increasing patient population.
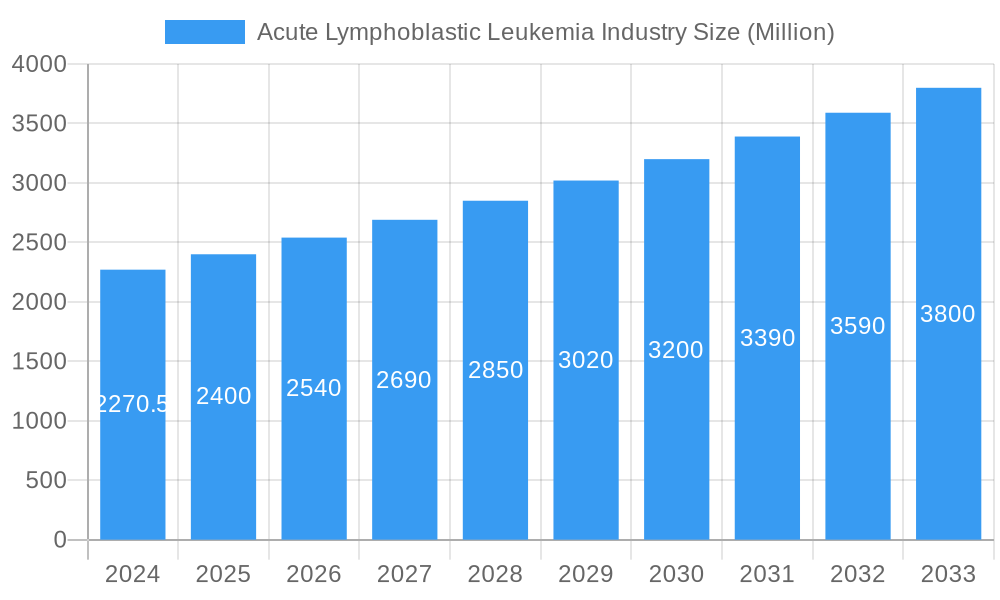
Acute Lymphoblastic Leukemia Industry Company Market Share

Gain unparalleled insights into the global Acute Lymphoblastic Leukemia (ALL) industry with our in-depth market analysis. This report delves into market dynamics, key segments, product innovations, and future growth trajectories from 2019 to 2033, with a base year of 2025. Driven by increasing ALL incidence and advancements in therapies, the Acute Lymphoblastic Leukemia market is poised for significant expansion. Explore critical data points, actionable strategies, and competitive landscapes to inform your business decisions in this vital oncology sector.
Acute Lymphoblastic Leukemia Industry Market Concentration & Dynamics
The Acute Lymphoblastic Leukemia industry exhibits a moderate to high market concentration, characterized by the presence of established pharmaceutical giants and emerging biotechs. Innovation ecosystems are robust, with significant investments in research and development for novel ALL treatments, particularly in targeted therapy and immunotherapy. Regulatory frameworks, governed by agencies like the FDA and EMA, play a crucial role in shaping market access and drug approvals. While traditional chemotherapy remains a cornerstone, the rise of targeted therapy and stem cell transplantation offers promising alternatives. End-user trends are shifting towards personalized medicine and less toxic treatment regimens. Merger and acquisition (M&A) activities, estimated at approximately 15-20 deals annually over the historical period, are driven by the pursuit of innovative pipelines and market expansion. Key players actively engage in strategic partnerships to accelerate drug development and commercialization.
Acute Lymphoblastic Leukemia Industry Industry Insights & Trends
The global Acute Lymphoblastic Leukemia industry is experiencing a transformative period, projected to grow from an estimated $15.5 billion in 2025 to over $28.2 billion by 2033, exhibiting a robust Compound Annual Growth Rate (CAGR) of approximately 7.9% during the forecast period. This substantial growth is propelled by several interconnected factors. Firstly, the increasing global incidence of Acute Lymphoblastic Leukemia, particularly in pediatric and young adult populations, continues to be a primary driver. Advancements in diagnostic technologies are leading to earlier and more accurate detection, subsequently increasing the patient pool seeking effective treatments.
Secondly, profound technological disruptions are revolutionizing ALL treatment paradigms. The shift from broad-spectrum chemotherapy to precision-driven approaches, including targeted therapy and innovative immunotherapies like CAR T-cell therapy, is a significant trend. These advancements offer improved efficacy and reduced toxicity, leading to better patient outcomes and higher adoption rates. The development of novel drug candidates targeting specific genetic mutations and pathways within B-cell ALL and T-cell ALL is a key area of focus for pharmaceutical companies. Furthermore, the integration of artificial intelligence (AI) in drug discovery and clinical trial optimization is accelerating the development of new ALL therapies.
Evolving consumer behaviors, influenced by increased awareness and a demand for personalized treatment plans, are also shaping the market. Patients and healthcare providers are increasingly seeking therapies that offer a higher quality of life and minimize long-term side effects. This preference is fueling the demand for targeted therapy and less invasive treatment options. The growing emphasis on patient-centric care and the development of supportive therapies to manage treatment side effects further contribute to market expansion. The Acute Lymphoblastic Leukemia market is therefore characterized by continuous innovation and a dynamic response to evolving patient needs and scientific breakthroughs.
Key Markets & Segments Leading Acute Lymphoblastic Leukemia Industry
The Acute Lymphoblastic Leukemia industry landscape is dominated by specific cell types and therapeutic approaches, reflecting the heterogeneity of the disease and the evolution of treatment strategies.
Dominant Segments:
Type of Cell:
- B-cell ALL represents the largest and most prevalent subtype, accounting for an estimated 75% of all ALL cases. This dominance is driven by its higher incidence in both pediatric and adult populations, making it a primary focus for drug development and market penetration. The availability of established treatment protocols and a robust pipeline of targeted therapies for B-cell ALL further solidifies its leading position.
- T-cell ALL, while less common, presents a more aggressive clinical course and often requires more intensive treatment regimens. The segment is witnessing significant research interest due to the unmet medical needs associated with its management, driving innovation in this area.
- Philadelphia chromosome-positive (Ph+) ALL is a distinct subtype characterized by the presence of the Philadelphia chromosome. Advancements in tyrosine kinase inhibitors (TKIs) have dramatically improved outcomes for Ph+ ALL patients, making this segment a crucial area of growth and innovation in targeted therapy.
Type of Therapy:
- Chemotherapy remains a foundational treatment modality for ALL, with established regimens like the Hyper - CVAD Regimen and CALGB 8811 Regimen widely employed. The Linker Regimen and various Nucleoside Inhibitors also play significant roles. The sheer volume of patients managed with chemotherapy ensures its continued market leadership.
- Targeted Therapy is emerging as a critical growth driver. The development of drugs specifically designed to target molecular abnormalities in ALL cells, such as TKIs for Ph+ ALL and antibody-drug conjugates (ADCs) for B-cell ALL, is revolutionizing treatment outcomes. The market share of targeted therapies is projected to expand significantly.
- Stem Cell Transplantation, including autologous and allogeneic transplantation, is a vital curative option for high-risk ALL patients. Its role in achieving long-term remission, particularly for relapsed or refractory cases, ensures its sustained importance in the therapeutic landscape.
- Radiation Therapy continues to play a role in specific scenarios, such as central nervous system prophylaxis or consolidation therapy, though its overall market share is less significant compared to chemotherapy and targeted therapies.
Drivers of Dominance:
- High Incidence: The significant prevalence of B-cell ALL ensures a large patient base for existing and emerging treatments.
- Unmet Medical Needs: The aggressive nature of T-cell ALL and the challenges in treating relapsed/refractory ALL create strong demand for innovative solutions.
- Technological Advancements: The development of highly effective targeted therapies and immunotherapies for specific ALL subtypes is a major catalyst for segment growth.
- Clinical Trial Successes: Positive outcomes from clinical trials for novel ALL drugs in B-cell ALL and T-cell ALL are driving market adoption and investment.
- Supportive Care Improvements: Enhancements in supportive care facilitate the use of more intensive treatment regimens, including stem cell transplantation.
Acute Lymphoblastic Leukemia Industry Product Developments
The Acute Lymphoblastic Leukemia industry is witnessing a rapid influx of innovative products, primarily focusing on targeted therapy and immunotherapy. Key developments include novel tyrosine kinase inhibitors (TKIs) for Philadelphia chromosome-positive (Ph+) ALL, significantly improving patient survival rates. Furthermore, advances in chimeric antigen receptor (CAR) T-cell therapy are revolutionizing the treatment of relapsed and refractory B-cell ALL, offering a potential cure for patients with limited options. Bispecific antibodies that engage the patient's immune system to target leukemia cells are also gaining traction. These advancements represent a paradigm shift from broad-spectrum chemotherapy towards personalized, mechanism-based treatments, offering improved efficacy and reduced toxicity for patients battling ALL.
Challenges in the Acute Lymphoblastic Leukemia Industry Market
Despite promising advancements, the Acute Lymphoblastic Leukemia industry faces significant challenges. High drug development costs and lengthy regulatory approval processes create substantial financial risks for pharmaceutical companies. The rarity of certain ALL subtypes can limit the market size for highly specialized therapies, impacting commercial viability. Furthermore, the emergence of drug resistance and the need for effective second-line and third-line treatment options remain persistent hurdles. Competitive pressures among key players and the pricing of novel, high-cost therapies can also pose access challenges for healthcare systems and patients, with the overall market estimated to be facing an approximate 10-15% challenge in reaching all eligible patient populations due to these factors.
Forces Driving Acute Lymphoblastic Leukemia Industry Growth
Several powerful forces are propelling the Acute Lymphoblastic Leukemia industry forward. Technological advancements in genomics and molecular diagnostics are enabling more precise identification of ALL subtypes and personalized treatment strategies, driving demand for targeted therapy. Increasing global incidence of ALL, particularly in emerging economies, is expanding the patient pool. Favorable regulatory pathways for orphan drugs and expedited review processes for life-saving oncology treatments are accelerating market entry. Moreover, significant investments in research and development by leading pharmaceutical companies, coupled with a growing awareness of the disease and its treatment options, are fostering continuous innovation and market growth.
Long-Term Growth Catalysts in the Acute Lymphoblastic Leukemia Industry Market
The long-term growth of the Acute Lymphoblastic Leukemia industry is underpinned by several key catalysts. Continued innovation in immunotherapy, including next-generation CAR T-cell therapies and novel antibody-based treatments, holds immense potential to further improve patient outcomes and expand treatment options for resistant disease. The development of more oral and less toxic targeted agents will enhance patient adherence and quality of life. Furthermore, the expansion of stem cell transplantation techniques and the exploration of ex-vivo gene editing for cellular therapies offer promising avenues for curative treatments. Strategic partnerships and collaborations between pharmaceutical companies, academic institutions, and research organizations will accelerate the translation of scientific discoveries into clinical applications, ensuring sustained growth and addressing unmet medical needs in ALL.
Emerging Opportunities in Acute Lymphoblastic Leukemia Industry
Emerging opportunities within the Acute Lymphoblastic Leukemia industry are abundant, driven by ongoing scientific breakthroughs and evolving healthcare landscapes. The development of novel therapeutic targets for T-cell ALL and relapsed/refractory B-cell ALL presents significant market potential. The increasing adoption of precision medicine approaches, leveraging advanced diagnostics to tailor treatment regimens, is creating demand for companion diagnostics and targeted agents. Expansion into underserved geographical markets, particularly in Asia-Pacific and Latin America, where ALL incidence is rising, offers substantial growth prospects. Furthermore, the exploration of combination therapies involving novel agents with established treatments, as well as the advancement of minimal residual disease (MRD) detection technologies, will open new avenues for improved patient management and therapeutic strategies.
Leading Players in the Acute Lymphoblastic Leukemia Industry Sector
- Genmab A/S
- Novartis AG
- F Hoffmann-La Roche Ltd
- Erytech Pharma
- Celgene Corporation
- Bristol Myers Squibb Company
- Sanofi SA
- Eisai Co Ltd
- GlaxoSmithKline PLC
- Pfizer Inc
Key Milestones in Acute Lymphoblastic Leukemia Industry Industry
- 2019: Approval of a new CAR T-cell therapy for relapsed/refractory B-cell ALL, marking a significant advancement in immunotherapy.
- 2020: Launch of an innovative tyrosine kinase inhibitor (TKI) demonstrating superior efficacy in Philadelphia chromosome-positive (Ph+) ALL.
- 2021: Significant clinical trial results published for a novel bispecific antibody targeting CD19 and CD3, showing high remission rates.
- 2022: Acquisition of a promising early-stage biotech company focused on developing novel ALL drug conjugates by a major pharmaceutical player.
- 2023: Regulatory submission for a new targeted therapy aimed at a specific genetic mutation prevalent in T-cell ALL.
- 2024: Increased investment in expanding manufacturing capacity for CAR T-cell therapies to meet growing demand.
Strategic Outlook for Acute Lymphoblastic Leukemia Industry Market
The strategic outlook for the Acute Lymphoblastic Leukemia industry is exceptionally promising, characterized by sustained innovation and market expansion. Key growth accelerators include the continued development and adoption of targeted therapies and immunotherapies, offering improved efficacy and quality of life for patients. Strategic partnerships and collaborations will be crucial for leveraging research breakthroughs and navigating complex regulatory pathways. The increasing focus on personalized medicine, supported by advancements in diagnostics, will drive demand for tailored treatment solutions. Furthermore, expanding access to novel therapies in emerging markets represents a significant untapped potential, promising substantial long-term growth and contributing to a brighter future for ALL patients worldwide.
Acute Lymphoblastic Leukemia Industry Segmentation
-
1. Type of Cell
- 1.1. B-cell ALL
- 1.2. T-cell ALL
- 1.3. Philadel
-
2. Type of Therapy
-
2.1. Chemotherapy
- 2.1.1. Hyper - CVAD Regimen
- 2.1.2. CALGB 8811 Regimen
- 2.1.3. Linker Regimen
- 2.1.4. Nucleoside Inhibitors
- 2.1.5. Other Types of Therapies
- 2.2. Targeted Therapy
- 2.3. Radiation Therapy
- 2.4. Stem Cell Transplantation
-
2.1. Chemotherapy
Acute Lymphoblastic Leukemia Industry Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. Europe
- 2.1. Germany
- 2.2. United Kingdom
- 2.3. France
- 2.4. Italy
- 2.5. Spain
- 2.6. Rest of Europe
-
3. Asia Pacific
- 3.1. China
- 3.2. Japan
- 3.3. India
- 3.4. Australia
- 3.5. South Korea
- 3.6. Rest of Asia Pacific
- 4. Middle East
-
5. GCC
- 5.1. South Africa
- 5.2. Rest of Middle East
-
6. South America
- 6.1. Brazil
- 6.2. Argentina
- 6.3. Rest of South America
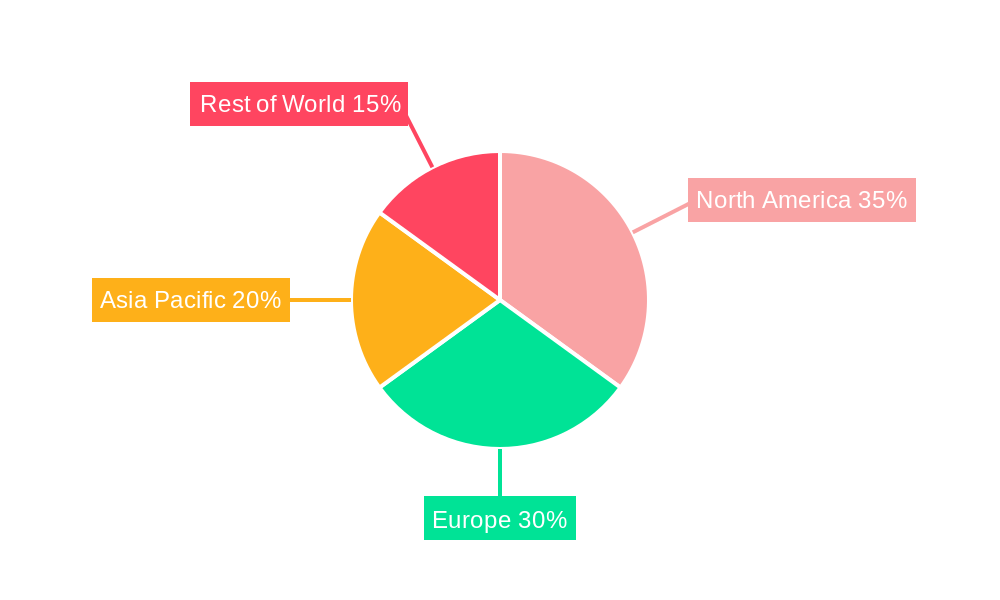
Acute Lymphoblastic Leukemia Industry Regional Market Share

Geographic Coverage of Acute Lymphoblastic Leukemia Industry
Acute Lymphoblastic Leukemia Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 5.61% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. ; Rise in the Incidences of Acute Lymphoblastic Leukemia; Increasing Initiatives Taken by the Government and Private Organizations
- 3.3. Market Restrains
- 3.3.1. ; Stringent Regulatory Pathways; High Cost Asscoiated with the Treatment
- 3.4. Market Trends
- 3.4.1. Chemotherapy is Expected to Dominate the Type of Therapy Segment
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Acute Lymphoblastic Leukemia Industry Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Type of Cell
- 5.1.1. B-cell ALL
- 5.1.2. T-cell ALL
- 5.1.3. Philadel
- 5.2. Market Analysis, Insights and Forecast - by Type of Therapy
- 5.2.1. Chemotherapy
- 5.2.1.1. Hyper - CVAD Regimen
- 5.2.1.2. CALGB 8811 Regimen
- 5.2.1.3. Linker Regimen
- 5.2.1.4. Nucleoside Inhibitors
- 5.2.1.5. Other Types of Therapies
- 5.2.2. Targeted Therapy
- 5.2.3. Radiation Therapy
- 5.2.4. Stem Cell Transplantation
- 5.2.1. Chemotherapy
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. Europe
- 5.3.3. Asia Pacific
- 5.3.4. Middle East
- 5.3.5. GCC
- 5.3.6. South America
- 5.1. Market Analysis, Insights and Forecast - by Type of Cell
- 6. North America Acute Lymphoblastic Leukemia Industry Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Type of Cell
- 6.1.1. B-cell ALL
- 6.1.2. T-cell ALL
- 6.1.3. Philadel
- 6.2. Market Analysis, Insights and Forecast - by Type of Therapy
- 6.2.1. Chemotherapy
- 6.2.1.1. Hyper - CVAD Regimen
- 6.2.1.2. CALGB 8811 Regimen
- 6.2.1.3. Linker Regimen
- 6.2.1.4. Nucleoside Inhibitors
- 6.2.1.5. Other Types of Therapies
- 6.2.2. Targeted Therapy
- 6.2.3. Radiation Therapy
- 6.2.4. Stem Cell Transplantation
- 6.2.1. Chemotherapy
- 6.1. Market Analysis, Insights and Forecast - by Type of Cell
- 7. Europe Acute Lymphoblastic Leukemia Industry Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Type of Cell
- 7.1.1. B-cell ALL
- 7.1.2. T-cell ALL
- 7.1.3. Philadel
- 7.2. Market Analysis, Insights and Forecast - by Type of Therapy
- 7.2.1. Chemotherapy
- 7.2.1.1. Hyper - CVAD Regimen
- 7.2.1.2. CALGB 8811 Regimen
- 7.2.1.3. Linker Regimen
- 7.2.1.4. Nucleoside Inhibitors
- 7.2.1.5. Other Types of Therapies
- 7.2.2. Targeted Therapy
- 7.2.3. Radiation Therapy
- 7.2.4. Stem Cell Transplantation
- 7.2.1. Chemotherapy
- 7.1. Market Analysis, Insights and Forecast - by Type of Cell
- 8. Asia Pacific Acute Lymphoblastic Leukemia Industry Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Type of Cell
- 8.1.1. B-cell ALL
- 8.1.2. T-cell ALL
- 8.1.3. Philadel
- 8.2. Market Analysis, Insights and Forecast - by Type of Therapy
- 8.2.1. Chemotherapy
- 8.2.1.1. Hyper - CVAD Regimen
- 8.2.1.2. CALGB 8811 Regimen
- 8.2.1.3. Linker Regimen
- 8.2.1.4. Nucleoside Inhibitors
- 8.2.1.5. Other Types of Therapies
- 8.2.2. Targeted Therapy
- 8.2.3. Radiation Therapy
- 8.2.4. Stem Cell Transplantation
- 8.2.1. Chemotherapy
- 8.1. Market Analysis, Insights and Forecast - by Type of Cell
- 9. Middle East Acute Lymphoblastic Leukemia Industry Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Type of Cell
- 9.1.1. B-cell ALL
- 9.1.2. T-cell ALL
- 9.1.3. Philadel
- 9.2. Market Analysis, Insights and Forecast - by Type of Therapy
- 9.2.1. Chemotherapy
- 9.2.1.1. Hyper - CVAD Regimen
- 9.2.1.2. CALGB 8811 Regimen
- 9.2.1.3. Linker Regimen
- 9.2.1.4. Nucleoside Inhibitors
- 9.2.1.5. Other Types of Therapies
- 9.2.2. Targeted Therapy
- 9.2.3. Radiation Therapy
- 9.2.4. Stem Cell Transplantation
- 9.2.1. Chemotherapy
- 9.1. Market Analysis, Insights and Forecast - by Type of Cell
- 10. GCC Acute Lymphoblastic Leukemia Industry Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Type of Cell
- 10.1.1. B-cell ALL
- 10.1.2. T-cell ALL
- 10.1.3. Philadel
- 10.2. Market Analysis, Insights and Forecast - by Type of Therapy
- 10.2.1. Chemotherapy
- 10.2.1.1. Hyper - CVAD Regimen
- 10.2.1.2. CALGB 8811 Regimen
- 10.2.1.3. Linker Regimen
- 10.2.1.4. Nucleoside Inhibitors
- 10.2.1.5. Other Types of Therapies
- 10.2.2. Targeted Therapy
- 10.2.3. Radiation Therapy
- 10.2.4. Stem Cell Transplantation
- 10.2.1. Chemotherapy
- 10.1. Market Analysis, Insights and Forecast - by Type of Cell
- 11. South America Acute Lymphoblastic Leukemia Industry Analysis, Insights and Forecast, 2020-2032
- 11.1. Market Analysis, Insights and Forecast - by Type of Cell
- 11.1.1. B-cell ALL
- 11.1.2. T-cell ALL
- 11.1.3. Philadel
- 11.2. Market Analysis, Insights and Forecast - by Type of Therapy
- 11.2.1. Chemotherapy
- 11.2.1.1. Hyper - CVAD Regimen
- 11.2.1.2. CALGB 8811 Regimen
- 11.2.1.3. Linker Regimen
- 11.2.1.4. Nucleoside Inhibitors
- 11.2.1.5. Other Types of Therapies
- 11.2.2. Targeted Therapy
- 11.2.3. Radiation Therapy
- 11.2.4. Stem Cell Transplantation
- 11.2.1. Chemotherapy
- 11.1. Market Analysis, Insights and Forecast - by Type of Cell
- 12. Competitive Analysis
- 12.1. Global Market Share Analysis 2025
- 12.2. Company Profiles
- 12.2.1 Genmab A/S
- 12.2.1.1. Overview
- 12.2.1.2. Products
- 12.2.1.3. SWOT Analysis
- 12.2.1.4. Recent Developments
- 12.2.1.5. Financials (Based on Availability)
- 12.2.2 Novartis AG
- 12.2.2.1. Overview
- 12.2.2.2. Products
- 12.2.2.3. SWOT Analysis
- 12.2.2.4. Recent Developments
- 12.2.2.5. Financials (Based on Availability)
- 12.2.3 F Hoffmann-La Roche Ltd
- 12.2.3.1. Overview
- 12.2.3.2. Products
- 12.2.3.3. SWOT Analysis
- 12.2.3.4. Recent Developments
- 12.2.3.5. Financials (Based on Availability)
- 12.2.4 Erytech Pharma
- 12.2.4.1. Overview
- 12.2.4.2. Products
- 12.2.4.3. SWOT Analysis
- 12.2.4.4. Recent Developments
- 12.2.4.5. Financials (Based on Availability)
- 12.2.5 Celegene Corporation
- 12.2.5.1. Overview
- 12.2.5.2. Products
- 12.2.5.3. SWOT Analysis
- 12.2.5.4. Recent Developments
- 12.2.5.5. Financials (Based on Availability)
- 12.2.6 Bristol Myer Squibb Company
- 12.2.6.1. Overview
- 12.2.6.2. Products
- 12.2.6.3. SWOT Analysis
- 12.2.6.4. Recent Developments
- 12.2.6.5. Financials (Based on Availability)
- 12.2.7 Sanofi SA*List Not Exhaustive
- 12.2.7.1. Overview
- 12.2.7.2. Products
- 12.2.7.3. SWOT Analysis
- 12.2.7.4. Recent Developments
- 12.2.7.5. Financials (Based on Availability)
- 12.2.8 Eisai Co Ltd
- 12.2.8.1. Overview
- 12.2.8.2. Products
- 12.2.8.3. SWOT Analysis
- 12.2.8.4. Recent Developments
- 12.2.8.5. Financials (Based on Availability)
- 12.2.9 GlaxoSmithKline PLC
- 12.2.9.1. Overview
- 12.2.9.2. Products
- 12.2.9.3. SWOT Analysis
- 12.2.9.4. Recent Developments
- 12.2.9.5. Financials (Based on Availability)
- 12.2.10 Pfizer Inc
- 12.2.10.1. Overview
- 12.2.10.2. Products
- 12.2.10.3. SWOT Analysis
- 12.2.10.4. Recent Developments
- 12.2.10.5. Financials (Based on Availability)
- 12.2.1 Genmab A/S
List of Figures
- Figure 1: Global Acute Lymphoblastic Leukemia Industry Revenue Breakdown (undefined, %) by Region 2025 & 2033
- Figure 2: North America Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Type of Cell 2025 & 2033
- Figure 3: North America Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Type of Cell 2025 & 2033
- Figure 4: North America Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Type of Therapy 2025 & 2033
- Figure 5: North America Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Type of Therapy 2025 & 2033
- Figure 6: North America Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Country 2025 & 2033
- Figure 7: North America Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Country 2025 & 2033
- Figure 8: Europe Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Type of Cell 2025 & 2033
- Figure 9: Europe Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Type of Cell 2025 & 2033
- Figure 10: Europe Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Type of Therapy 2025 & 2033
- Figure 11: Europe Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Type of Therapy 2025 & 2033
- Figure 12: Europe Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Country 2025 & 2033
- Figure 13: Europe Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Country 2025 & 2033
- Figure 14: Asia Pacific Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Type of Cell 2025 & 2033
- Figure 15: Asia Pacific Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Type of Cell 2025 & 2033
- Figure 16: Asia Pacific Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Type of Therapy 2025 & 2033
- Figure 17: Asia Pacific Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Type of Therapy 2025 & 2033
- Figure 18: Asia Pacific Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Country 2025 & 2033
- Figure 19: Asia Pacific Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Country 2025 & 2033
- Figure 20: Middle East Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Type of Cell 2025 & 2033
- Figure 21: Middle East Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Type of Cell 2025 & 2033
- Figure 22: Middle East Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Type of Therapy 2025 & 2033
- Figure 23: Middle East Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Type of Therapy 2025 & 2033
- Figure 24: Middle East Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Country 2025 & 2033
- Figure 25: Middle East Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Country 2025 & 2033
- Figure 26: GCC Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Type of Cell 2025 & 2033
- Figure 27: GCC Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Type of Cell 2025 & 2033
- Figure 28: GCC Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Type of Therapy 2025 & 2033
- Figure 29: GCC Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Type of Therapy 2025 & 2033
- Figure 30: GCC Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Country 2025 & 2033
- Figure 31: GCC Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Country 2025 & 2033
- Figure 32: South America Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Type of Cell 2025 & 2033
- Figure 33: South America Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Type of Cell 2025 & 2033
- Figure 34: South America Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Type of Therapy 2025 & 2033
- Figure 35: South America Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Type of Therapy 2025 & 2033
- Figure 36: South America Acute Lymphoblastic Leukemia Industry Revenue (undefined), by Country 2025 & 2033
- Figure 37: South America Acute Lymphoblastic Leukemia Industry Revenue Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Type of Cell 2020 & 2033
- Table 2: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Type of Therapy 2020 & 2033
- Table 3: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Region 2020 & 2033
- Table 4: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Type of Cell 2020 & 2033
- Table 5: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Type of Therapy 2020 & 2033
- Table 6: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Country 2020 & 2033
- Table 7: United States Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 8: Canada Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 9: Mexico Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 10: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Type of Cell 2020 & 2033
- Table 11: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Type of Therapy 2020 & 2033
- Table 12: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Country 2020 & 2033
- Table 13: Germany Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 14: United Kingdom Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 15: France Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 16: Italy Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 17: Spain Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 18: Rest of Europe Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 19: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Type of Cell 2020 & 2033
- Table 20: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Type of Therapy 2020 & 2033
- Table 21: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Country 2020 & 2033
- Table 22: China Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 23: Japan Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 24: India Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 25: Australia Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 26: South Korea Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 27: Rest of Asia Pacific Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 28: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Type of Cell 2020 & 2033
- Table 29: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Type of Therapy 2020 & 2033
- Table 30: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Country 2020 & 2033
- Table 31: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Type of Cell 2020 & 2033
- Table 32: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Type of Therapy 2020 & 2033
- Table 33: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Country 2020 & 2033
- Table 34: South Africa Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 35: Rest of Middle East Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 36: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Type of Cell 2020 & 2033
- Table 37: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Type of Therapy 2020 & 2033
- Table 38: Global Acute Lymphoblastic Leukemia Industry Revenue undefined Forecast, by Country 2020 & 2033
- Table 39: Brazil Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 40: Argentina Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 41: Rest of South America Acute Lymphoblastic Leukemia Industry Revenue (undefined) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Acute Lymphoblastic Leukemia Industry?
The projected CAGR is approximately 5.61%.
2. Which companies are prominent players in the Acute Lymphoblastic Leukemia Industry?
Key companies in the market include Genmab A/S, Novartis AG, F Hoffmann-La Roche Ltd, Erytech Pharma, Celegene Corporation, Bristol Myer Squibb Company, Sanofi SA*List Not Exhaustive, Eisai Co Ltd, GlaxoSmithKline PLC, Pfizer Inc.
3. What are the main segments of the Acute Lymphoblastic Leukemia Industry?
The market segments include Type of Cell, Type of Therapy.
4. Can you provide details about the market size?
The market size is estimated to be USD XXX N/A as of 2022.
5. What are some drivers contributing to market growth?
; Rise in the Incidences of Acute Lymphoblastic Leukemia; Increasing Initiatives Taken by the Government and Private Organizations.
6. What are the notable trends driving market growth?
Chemotherapy is Expected to Dominate the Type of Therapy Segment.
7. Are there any restraints impacting market growth?
; Stringent Regulatory Pathways; High Cost Asscoiated with the Treatment.
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in N/A.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Acute Lymphoblastic Leukemia Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Acute Lymphoblastic Leukemia Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Acute Lymphoblastic Leukemia Industry?
To stay informed about further developments, trends, and reports in the Acute Lymphoblastic Leukemia Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
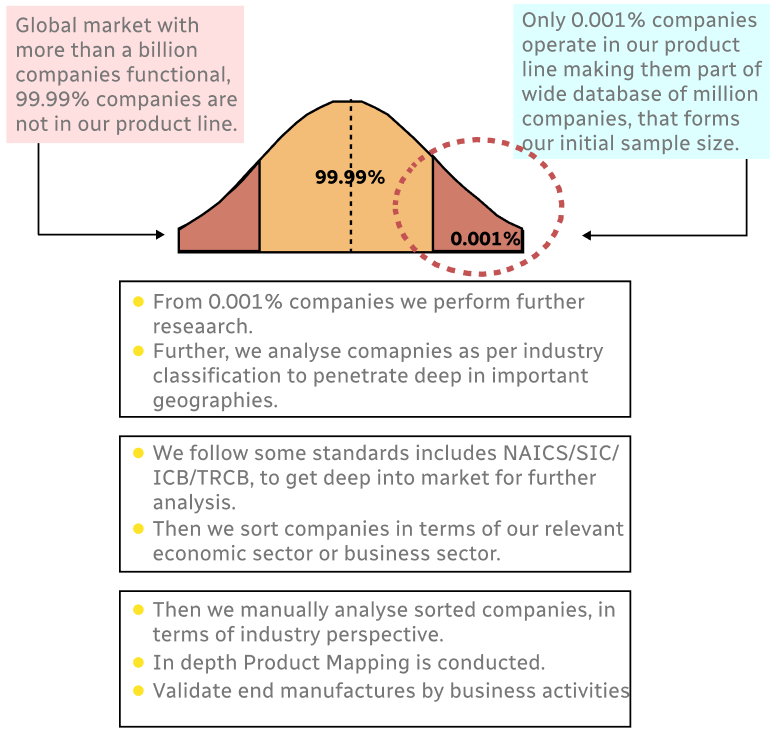
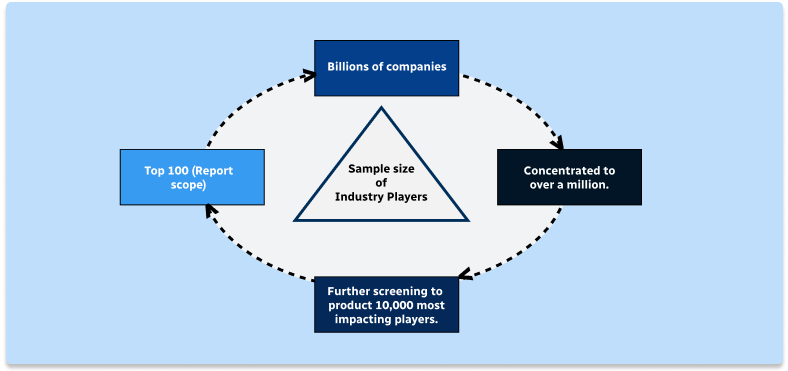
Step 1 - Identification of Relevant Samples Size from Population Database
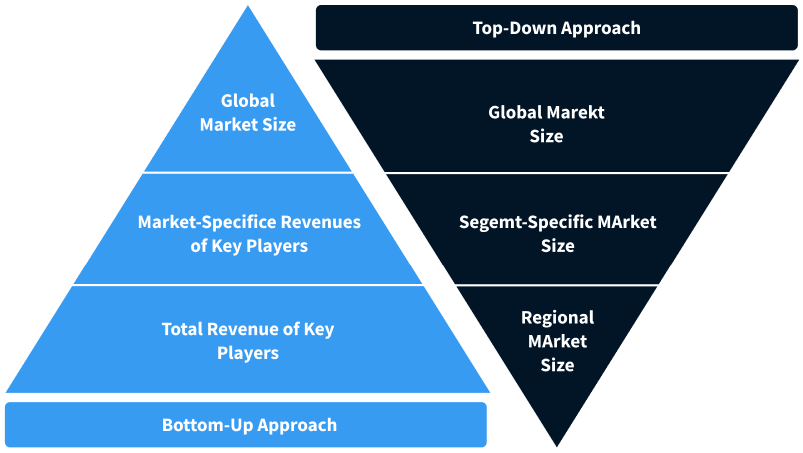
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


