Key Insights
The Chilean cardiovascular devices market is poised for significant growth, with a projected market size of $72.22 billion in 2025 and an estimated CAGR of 6.4% from 2025 to 2033. This robust expansion is driven by a confluence of factors, including the increasing prevalence of cardiovascular diseases (CVDs) in Chile, a growing aging population, and rising healthcare expenditure. As the population ages, the demand for diagnostic and monitoring devices like electrocardiogram (ECG) machines and remote cardiac monitoring systems is expected to surge. Simultaneously, advancements in therapeutic and surgical devices, such as innovative cardiac rhythm management devices, sophisticated catheters, and advanced heart valves and stents, are crucial in managing and treating complex cardiac conditions. The Chilean government's focus on improving healthcare infrastructure and increasing access to advanced medical technologies further bolsters market prospects. Furthermore, a growing awareness among the populace regarding the importance of cardiac health and early detection of CVDs contributes to a higher demand for these life-saving devices.
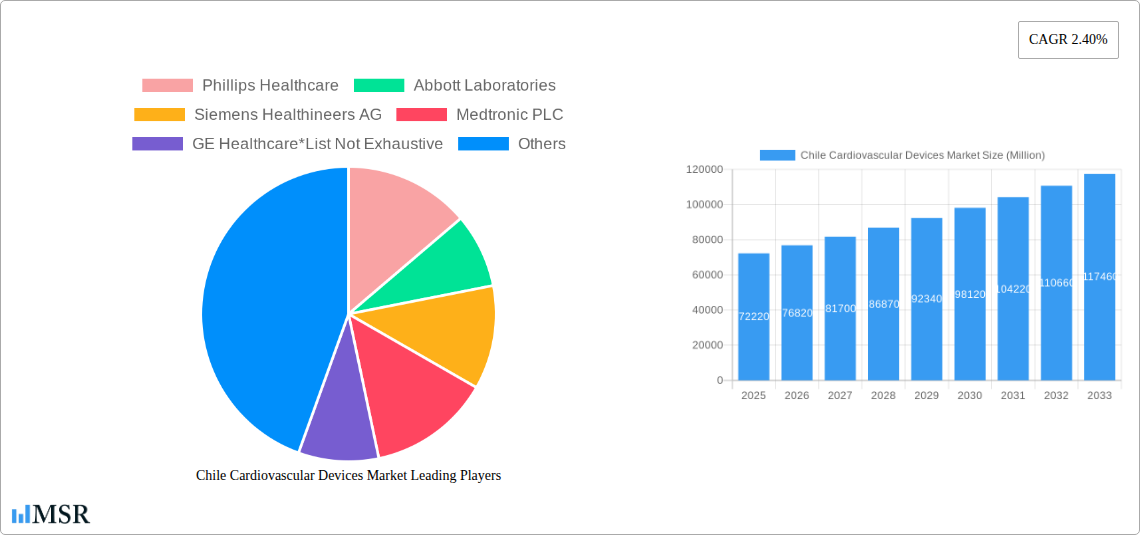
Chile Cardiovascular Devices Market Market Size (In Billion)

The market's expansion will be significantly influenced by ongoing technological innovations and the adoption of minimally invasive procedures. Key players such as Phillips Healthcare, Abbott Laboratories, Siemens Healthineers AG, Medtronic PLC, and GE Healthcare are actively investing in research and development to introduce next-generation cardiovascular devices. These innovations aim to enhance patient outcomes, reduce recovery times, and improve the overall efficiency of cardiac care. While the market is experiencing strong growth, certain restraints might emerge, such as the high cost of some advanced devices and the need for specialized training for healthcare professionals. However, the overarching trend points towards a dynamic and expanding Chilean cardiovascular devices market, offering substantial opportunities for stakeholders and improved cardiac care for the Chilean population. The forecast period of 2025-2033 is anticipated to witness a substantial uplift in market value, driven by these synergistic forces.
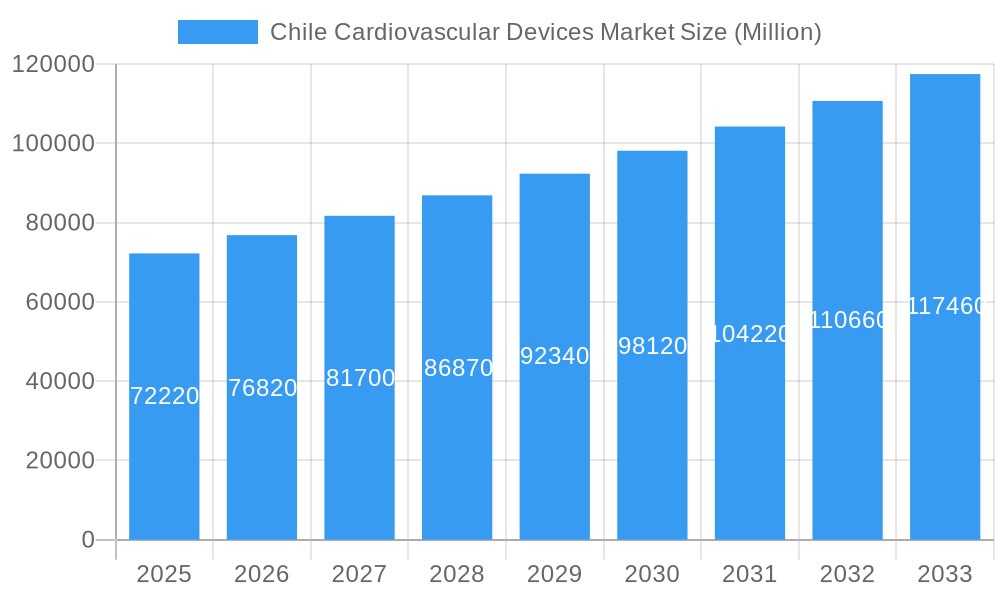
Chile Cardiovascular Devices Market Company Market Share

Here's the SEO-optimized report description for the Chile Cardiovascular Devices Market, designed for high search visibility and to attract industry stakeholders without any further modifications:
Chile Cardiovascular Devices Market: In-depth Analysis & Future Outlook (2019-2033)
Gain comprehensive intelligence on the burgeoning Chile Cardiovascular Devices Market with this essential report. Spanning from 2019 to 2033, this analysis provides a critical look at the market size, growth drivers, key segments, and leading players shaping the future of cardiovascular care in Chile. Understand the dynamics of cardiac rhythm management devices, cardiac assist devices, stents, heart valves, and advanced diagnostic and monitoring devices. This report is crucial for medical device manufacturers, healthcare providers, investors, and policymakers seeking to capitalize on the estimated USD XX billion market by 2033.
Chile Cardiovascular Devices Market Market Concentration & Dynamics
The Chile Cardiovascular Devices Market exhibits a moderate level of concentration, with key global players like Abbott Laboratories, Medtronic PLC, Philips Healthcare, and Siemens Healthineers AG holding significant market share. These companies drive innovation and penetrate the market through extensive distribution networks and strategic partnerships. The innovation ecosystem is fueled by ongoing research and development, particularly in minimally invasive technologies and remote patient monitoring solutions. Regulatory frameworks, overseen by the Chilean Ministry of Health, ensure product safety and efficacy, though navigating these requirements can pose challenges for smaller entrants. The threat of substitute products is relatively low in the cardiovascular device sector due to the highly specialized nature of the technologies. End-user trends are increasingly favoring devices that offer enhanced patient convenience, remote monitoring capabilities, and improved clinical outcomes. Merger and acquisition (M&A) activities are observed as companies seek to expand their portfolios and market reach. For instance, the last five years have seen an average of X M&A deals annually, primarily focused on acquiring innovative startups and expanding geographical presence. Key players' market share ranges from XX% for the leading player to X% for the top five combined.
Chile Cardiovascular Devices Market Industry Insights & Trends
The Chile Cardiovascular Devices Market is poised for robust growth, projected to reach an estimated USD XX billion by 2033, with a Compound Annual Growth Rate (CAGR) of X.XX% during the forecast period (2025–2033). This expansion is primarily driven by the increasing prevalence of cardiovascular diseases (CVDs) in Chile, a trend exacerbated by lifestyle changes, aging demographics, and a growing obese population. Technological disruptions are playing a pivotal role, with advancements in minimally invasive surgical techniques, sophisticated diagnostic imaging, and intelligent implantable devices transforming patient care. The adoption of remote cardiac monitoring solutions is rapidly gaining traction, allowing for continuous patient surveillance, early detection of anomalies, and reduced hospital readmissions. Furthermore, the integration of artificial intelligence (AI) and machine learning (ML) into cardiovascular devices promises enhanced diagnostic accuracy and personalized treatment plans. Evolving consumer behaviors are also influencing market dynamics, as patients become more informed and actively involved in their healthcare decisions, demanding solutions that offer convenience, improved quality of life, and greater control over their health. The shift towards value-based healthcare models is encouraging the adoption of devices that demonstrate clear clinical and economic benefits, such as those that reduce overall healthcare expenditure by preventing complications and hospitalizations. The penetration of advanced catheter technologies for complex interventions and the increasing demand for heart valves and stents are further contributing to market expansion. The focus on preventative care and early diagnosis, coupled with government initiatives to improve healthcare infrastructure, creates a fertile ground for the growth of the cardiovascular devices sector in Chile. The market size in the base year of 2025 is estimated at USD XX billion.
Key Markets & Segments Leading Chile Cardiovascular Devices Market
The Chile Cardiovascular Devices Market is significantly influenced by its distinct segments, with Therapeutic and Surgical Devices emerging as the dominant force, accounting for an estimated XX% of the total market value. Within this segment, Cardiac Rhythm Management Devices and Stents are particularly robust, driven by the rising incidence of arrhythmias and coronary artery disease. The increasing number of percutaneous coronary interventions (PCIs) fuels the demand for high-quality stents, while the aging population and growing awareness of atrial fibrillation contribute to the uptake of pacemakers and defibrillators.
Diagnostic and Monitoring Devices: This segment, while smaller in value, plays a crucial role in early detection and management.
- Electrocardiogram (ECG) Devices: Demand for both portable and advanced hospital-based ECG machines remains strong, driven by the need for routine cardiac assessment and emergency diagnostics.
- Remote Cardiac Monitoring: This sub-segment is experiencing exponential growth due to its ability to improve patient outcomes and reduce healthcare costs. The widespread adoption of wearable technology and cloud-based platforms further accelerates its expansion.
- Other Diagnostic and Monitoring Devices: This includes devices like Holter monitors, event recorders, and diagnostic imaging equipment, all essential for comprehensive cardiovascular assessment.
Therapeutic and Surgical Devices: This segment holds the largest market share, characterized by high-value, technologically advanced products.
- Cardiac Assist Devices: While a niche, the market for devices like ventricular assist devices (VADs) is growing, driven by advancements in heart failure treatment.
- Cardiac Rhythm Management Device: This is a cornerstone of the segment, encompassing pacemakers, implantable cardioverter-defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices.
- Catheter: Both diagnostic and interventional catheters are in high demand, especially those used in minimally invasive procedures like angioplasty and TAVI (Transcatheter Aortic Valve Implantation).
- Grafts: Vascular grafts are crucial for bypass surgeries and peripheral interventions.
- Heart Valves: The increasing prevalence of valvular heart disease and the growing preference for minimally invasive valve replacements (e.g., TAVR) are significant growth drivers.
- Stents: Drug-eluting stents (DES) dominate the market, with ongoing innovations in bioresorbable and drug-coated technologies.
- Other Therapeutic and Surgical Devices: This encompasses a range of products used in cardiovascular surgery and intervention.
The dominance of the Therapeutic and Surgical Devices segment is further bolstered by economic growth in Chile, which enables greater investment in advanced medical technologies and infrastructure. Government initiatives aimed at improving public health outcomes and expanding access to quality healthcare services also contribute significantly to the market’s strength in this area.
Chile Cardiovascular Devices Market Product Developments
Product innovations in the Chile Cardiovascular Devices Market are rapidly enhancing treatment efficacy and patient experience. Advances in stent technology, including the development of bioresorbable scaffolds and improved drug-eluting coatings, offer better long-term outcomes. The realm of heart valves is witnessing significant progress with the miniaturization and enhanced functionality of transcatheter aortic valve replacement (TAVR) systems, making them accessible to a wider patient population. Furthermore, the evolution of cardiac rhythm management devices includes smaller, longer-lasting pacemakers and sophisticated defibrillators with enhanced diagnostic capabilities. Remote cardiac monitoring solutions are becoming more intelligent, leveraging AI to provide real-time alerts and predictive analytics. These technological advancements are crucial for maintaining a competitive edge and addressing the growing demand for minimally invasive and patient-centric cardiovascular care.
Challenges in the Chile Cardiovascular Devices Market Market
The Chile Cardiovascular Devices Market faces several challenges that could impede its growth trajectory. Stringent regulatory approval processes can lead to delays in product launches, impacting market entry for new innovations. High costs associated with advanced cardiovascular devices and their implantation procedures can create affordability issues for a significant portion of the population, limiting market penetration, especially in public healthcare settings. Supply chain disruptions, exacerbated by global events, can lead to product shortages and price volatility. Intense competition among established players and emerging local manufacturers also puts pressure on profit margins. Furthermore, a shortage of highly skilled electrophysiologists and cardiac surgeons can limit the adoption of complex new technologies.
Forces Driving Chile Cardiovascular Devices Market Growth
The Chile Cardiovascular Devices Market is propelled by several key forces. A primary driver is the escalating burden of cardiovascular diseases, stemming from an aging population, increasing rates of obesity and diabetes, and lifestyle factors. Technological advancements, particularly in minimally invasive techniques and smart devices, are expanding treatment options and improving patient outcomes. Government initiatives focused on improving healthcare infrastructure and increasing access to quality medical care also play a crucial role. Growing patient awareness and demand for advanced, less invasive procedures further fuel market expansion. The increasing disposable income in certain segments of the Chilean population also contributes to the demand for premium cardiovascular devices.
Challenges in the Chile Cardiovascular Devices Market Market
Long-term growth in the Chile Cardiovascular Devices Market hinges on overcoming persistent challenges. While technological adoption is a driver, ensuring widespread reimbursement and affordability for advanced cardiac assist devices, heart valves, and sophisticated diagnostic and monitoring devices remains critical. Building and expanding the skilled healthcare workforce, including interventional cardiologists and cardiac surgeons, is essential to support the uptake of complex procedures. Continuous investment in robust clinical evidence generation will be necessary to validate the long-term efficacy and cost-effectiveness of new technologies, thereby garnering trust from payers and providers. Addressing potential cybersecurity vulnerabilities in connected medical devices is also a growing concern.
Emerging Opportunities in Chile Cardiovascular Devices Market
Emerging opportunities in the Chile Cardiovascular Devices Market are abundant. The significant growth in the adoption of remote cardiac monitoring and telehealth solutions presents a substantial avenue for market expansion, catering to patient convenience and efficient healthcare delivery. The increasing focus on preventative cardiology and early disease detection offers a fertile ground for innovative diagnostic and monitoring devices, including wearable sensors and AI-powered diagnostic tools. As Chile's economy grows, so does the demand for premium cardiovascular treatments, opening doors for advanced therapeutic and surgical devices and specialized procedures. Furthermore, the development of personalized medicine approaches in cardiology, leveraging genetic and lifestyle data, presents a future frontier for tailored device solutions.
Leading Players in the Chile Cardiovascular Devices Market Sector
- Abbott Laboratories
- Medtronic PLC
- Philips Healthcare
- Siemens Healthineers AG
- GE Healthcare
- Welch Allyn Inc
- Boston Scientific Corporation
- Nihon Kohden Corporation
Key Milestones in Chile Cardiovascular Devices Market Industry
- 2019: Increased adoption of remote patient monitoring for chronic cardiovascular conditions, driven by technological advancements.
- 2020: Expansion of minimally invasive TAVI procedures, signaling a shift towards less invasive heart valve replacement.
- 2021: Growing investment in AI-powered diagnostic tools for cardiology, enhancing diagnostic accuracy.
- 2022: Surge in demand for advanced stents with improved drug-eluting technologies.
- 2023: Introduction of next-generation cardiac rhythm management devices with extended battery life and enhanced connectivity.
- 2024: Increased focus on patient-centric care models, driving the demand for wearable diagnostic and monitoring devices.
Strategic Outlook for Chile Cardiovascular Devices Market Market
The strategic outlook for the Chile Cardiovascular Devices Market is highly promising, fueled by continued demographic shifts and ongoing technological innovation. Key growth accelerators include the further integration of AI and big data analytics into cardiovascular devices, enabling predictive diagnostics and personalized treatment. Expanding the reach of minimally invasive procedures, particularly for heart valves and complex coronary interventions, will be critical. Strategic partnerships between device manufacturers, healthcare providers, and research institutions will be instrumental in driving innovation and facilitating market access. Investing in the training and development of healthcare professionals to support the adoption of cutting-edge technologies will also be a vital growth catalyst. The market's future success lies in its ability to provide accessible, effective, and patient-centric solutions to address the growing cardiovascular disease burden.
Chile Cardiovascular Devices Market Segmentation
-
1. Device Type
-
1.1. Diagnostic and Monitoring Devices
- 1.1.1. Electrocardiogram (ECG)
- 1.1.2. Remote Cardiac Monitoring
- 1.1.3. Other Diagnostic and Monitoring Devices
-
1.2. Therapeutic and Surgical Devices
- 1.2.1. Cardiac Assist Devices
- 1.2.2. Cardiac Rhythm Management Device
- 1.2.3. Catheter
- 1.2.4. Grafts
- 1.2.5. Heart Valves
- 1.2.6. Stents
- 1.2.7. Other Therapeutic and Surgical Devices
-
1.1. Diagnostic and Monitoring Devices
Chile Cardiovascular Devices Market Segmentation By Geography
- 1. Chile
Chile Cardiovascular Devices Market Regional Market Share

Geographic Coverage of Chile Cardiovascular Devices Market
Chile Cardiovascular Devices Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 6.5% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. ; Increasing Burden of Cardiovascular Diseases; Increased Preference of Minimally Invasive Procedures
- 3.3. Market Restrains
- 3.3.1. ; Stringent Regulatory Policies; High Cost of Instruments and Procedures
- 3.4. Market Trends
- 3.4.1. Electrocardiogram (ECG) is Expected to Dominate the Diagnostic And Monitoring Segment Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Chile Cardiovascular Devices Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 5.1.1. Diagnostic and Monitoring Devices
- 5.1.1.1. Electrocardiogram (ECG)
- 5.1.1.2. Remote Cardiac Monitoring
- 5.1.1.3. Other Diagnostic and Monitoring Devices
- 5.1.2. Therapeutic and Surgical Devices
- 5.1.2.1. Cardiac Assist Devices
- 5.1.2.2. Cardiac Rhythm Management Device
- 5.1.2.3. Catheter
- 5.1.2.4. Grafts
- 5.1.2.5. Heart Valves
- 5.1.2.6. Stents
- 5.1.2.7. Other Therapeutic and Surgical Devices
- 5.1.1. Diagnostic and Monitoring Devices
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. Chile
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 6. Competitive Analysis
- 6.1. Market Share Analysis 2025
- 6.2. Company Profiles
- 6.2.1 Phillips Healthcare
- 6.2.1.1. Overview
- 6.2.1.2. Products
- 6.2.1.3. SWOT Analysis
- 6.2.1.4. Recent Developments
- 6.2.1.5. Financials (Based on Availability)
- 6.2.2 Abbott Laboratories
- 6.2.2.1. Overview
- 6.2.2.2. Products
- 6.2.2.3. SWOT Analysis
- 6.2.2.4. Recent Developments
- 6.2.2.5. Financials (Based on Availability)
- 6.2.3 Siemens Healthineers AG
- 6.2.3.1. Overview
- 6.2.3.2. Products
- 6.2.3.3. SWOT Analysis
- 6.2.3.4. Recent Developments
- 6.2.3.5. Financials (Based on Availability)
- 6.2.4 Medtronic PLC
- 6.2.4.1. Overview
- 6.2.4.2. Products
- 6.2.4.3. SWOT Analysis
- 6.2.4.4. Recent Developments
- 6.2.4.5. Financials (Based on Availability)
- 6.2.5 GE Healthcare*List Not Exhaustive
- 6.2.5.1. Overview
- 6.2.5.2. Products
- 6.2.5.3. SWOT Analysis
- 6.2.5.4. Recent Developments
- 6.2.5.5. Financials (Based on Availability)
- 6.2.6 Welch Allyn Inc
- 6.2.6.1. Overview
- 6.2.6.2. Products
- 6.2.6.3. SWOT Analysis
- 6.2.6.4. Recent Developments
- 6.2.6.5. Financials (Based on Availability)
- 6.2.7 Boston Scientific Corporation
- 6.2.7.1. Overview
- 6.2.7.2. Products
- 6.2.7.3. SWOT Analysis
- 6.2.7.4. Recent Developments
- 6.2.7.5. Financials (Based on Availability)
- 6.2.8 Nihon Kohden Corporation
- 6.2.8.1. Overview
- 6.2.8.2. Products
- 6.2.8.3. SWOT Analysis
- 6.2.8.4. Recent Developments
- 6.2.8.5. Financials (Based on Availability)
- 6.2.1 Phillips Healthcare
List of Figures
- Figure 1: Chile Cardiovascular Devices Market Revenue Breakdown (undefined, %) by Product 2025 & 2033
- Figure 2: Chile Cardiovascular Devices Market Share (%) by Company 2025
List of Tables
- Table 1: Chile Cardiovascular Devices Market Revenue undefined Forecast, by Device Type 2020 & 2033
- Table 2: Chile Cardiovascular Devices Market Revenue undefined Forecast, by Region 2020 & 2033
- Table 3: Chile Cardiovascular Devices Market Revenue undefined Forecast, by Device Type 2020 & 2033
- Table 4: Chile Cardiovascular Devices Market Revenue undefined Forecast, by Country 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Chile Cardiovascular Devices Market?
The projected CAGR is approximately 6.5%.
2. Which companies are prominent players in the Chile Cardiovascular Devices Market?
Key companies in the market include Phillips Healthcare, Abbott Laboratories, Siemens Healthineers AG, Medtronic PLC, GE Healthcare*List Not Exhaustive, Welch Allyn Inc, Boston Scientific Corporation, Nihon Kohden Corporation.
3. What are the main segments of the Chile Cardiovascular Devices Market?
The market segments include Device Type.
4. Can you provide details about the market size?
The market size is estimated to be USD XXX N/A as of 2022.
5. What are some drivers contributing to market growth?
; Increasing Burden of Cardiovascular Diseases; Increased Preference of Minimally Invasive Procedures.
6. What are the notable trends driving market growth?
Electrocardiogram (ECG) is Expected to Dominate the Diagnostic And Monitoring Segment Over the Forecast Period.
7. Are there any restraints impacting market growth?
; Stringent Regulatory Policies; High Cost of Instruments and Procedures.
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in N/A.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Chile Cardiovascular Devices Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Chile Cardiovascular Devices Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Chile Cardiovascular Devices Market?
To stay informed about further developments, trends, and reports in the Chile Cardiovascular Devices Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
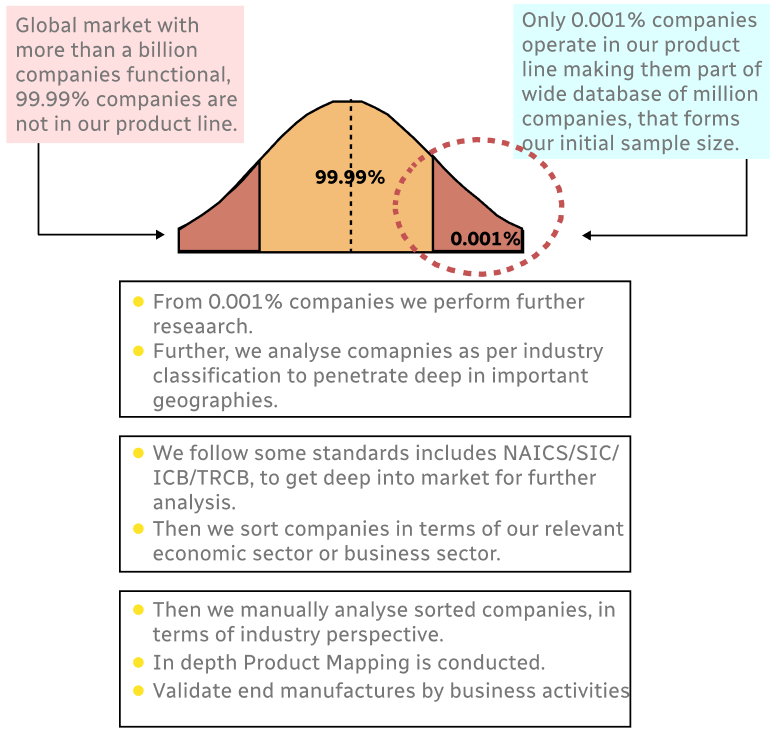
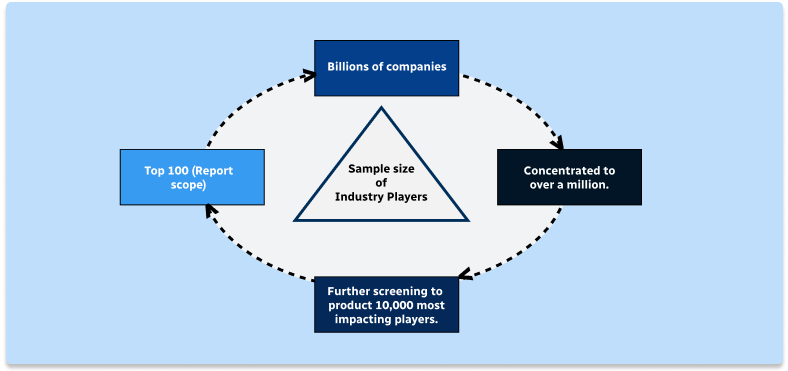
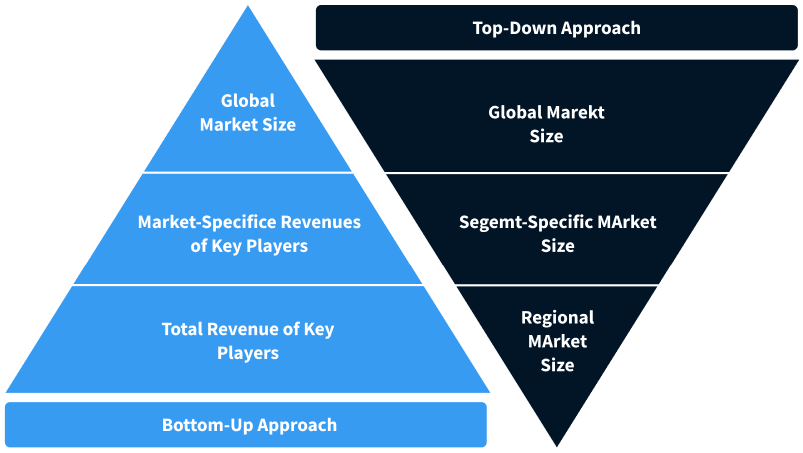
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
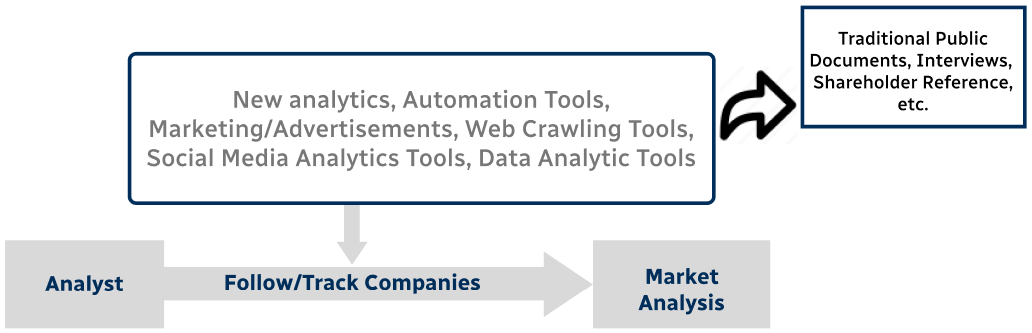
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


