Key Insights
The Circulating Tumor Cells (CTC) market is set for significant expansion, driven by enhanced adoption in early cancer detection, treatment monitoring, and personalized medicine. With an estimated market size of $14.04 billion in the base year 2025, the market is projected to grow at a Compound Annual Growth Rate (CAGR) of 13.92% through 2033. Key growth factors include advancements in CTC enrichment and detection technologies, rising global cancer prevalence, and an increasing emphasis on minimally invasive diagnostics. Expanding applications in understanding disease progression, identifying metastatic potential, and guiding therapeutic interventions are also bolstering market demand. Significant research and development investments by leading companies are further propelling innovation and market penetration.
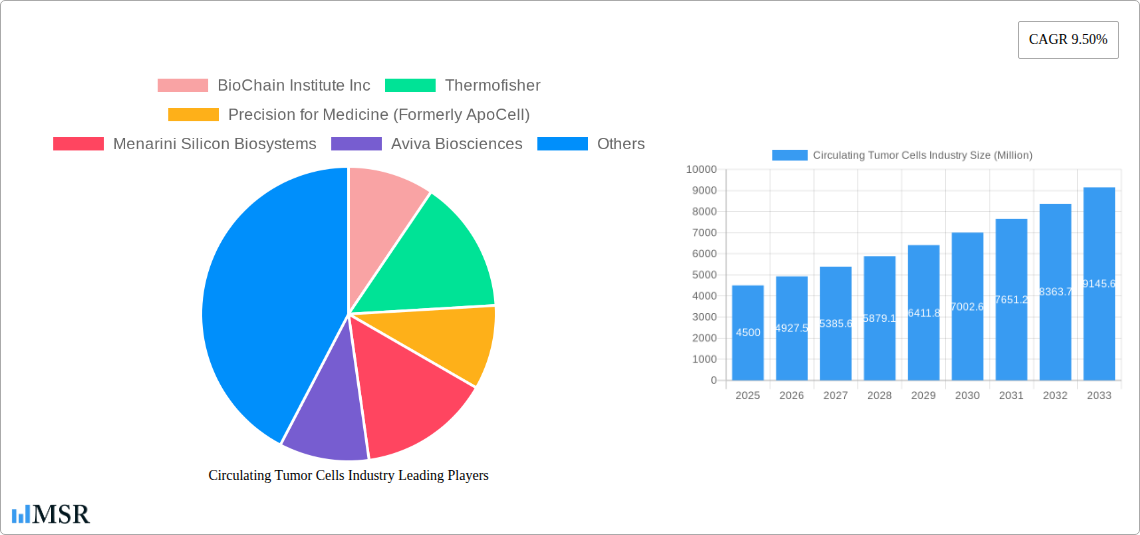
Circulating Tumor Cells Industry Market Size (In Billion)

The competitive landscape is characterized by strategic collaborations, product launches, and mergers and acquisitions. North America currently leads the market due to high healthcare expenditure and advanced diagnostic infrastructure. However, the Asia Pacific region is anticipated to witness the fastest growth, driven by rising cancer incidence and improving healthcare access. Challenges include the high cost of advanced CTC analysis platforms and the need for standardization. Nevertheless, the trend towards precision oncology and the potential of CTCs as a liquid biopsy solution for non-invasive cancer management are expected to drive sustained market growth.
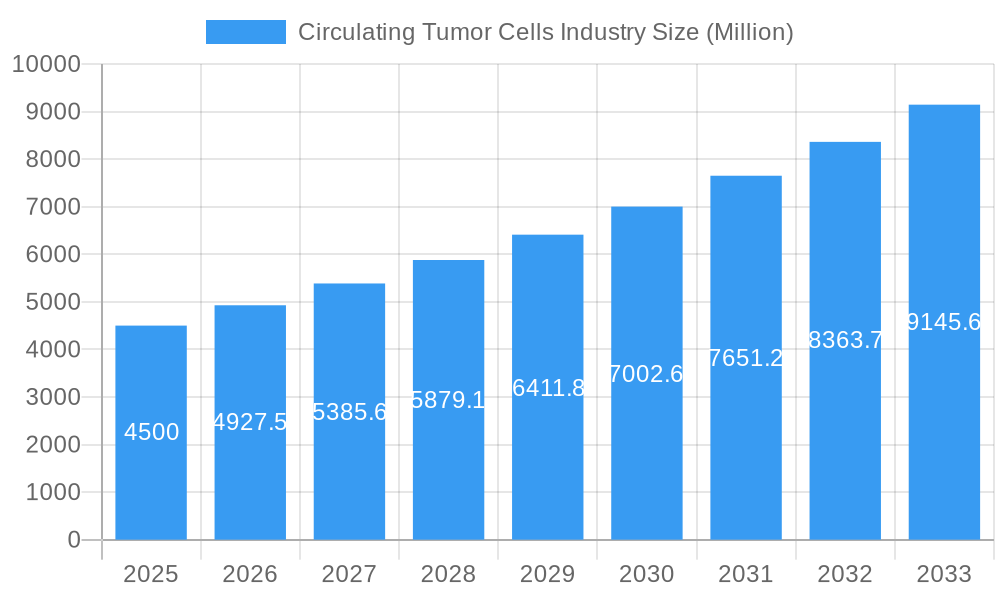
Circulating Tumor Cells Industry Company Market Share

This report offers a comprehensive analysis and forecast for the Circulating Tumor Cells (CTC) Industry, focusing on precision oncology market dynamics and future trends.
Circulating Tumor Cells (CTC) Industry: Precision Oncology Market Analysis & Forecast (2019–2033)
Explore the dynamic Circulating Tumor Cells (CTC) industry with our detailed market analysis and forecast. This report delivers critical insights into the evolving landscape of liquid biopsy, cancer diagnostics, and precision oncology, highlighting market dynamics, technological innovations, and strategic opportunities in circulating tumor cell detection for cancer patient management.
This report covers:
Key market segments analyzed:
The global CTC market is experiencing significant growth, fueled by advancements in early cancer detection, treatment monitoring, and the growing demand for personalized medicine. This report provides a granular view of market concentration, competitive landscapes, and the impact of regulatory frameworks.
- Study Period: 2019–2033
- Base Year: 2025
- Forecast Period: 2025–2033
- Technology: CTC Enrichment Methods (Positive Enrichment, Negative Enrichment, Others), CTC Detection Methods (Immunocytochemical, Molecular (RNA)-based, Others)
- Application: Multiple Chromosome Abnormalities, RNA Profiling, Protein Expression, Cellular Communication, Others
Circulating Tumor Cells Industry Market Concentration & Dynamics
The Circulating Tumor Cells (CTC) industry exhibits a moderate market concentration, with key players investing heavily in research and development to enhance CTC isolation and CTC analysis technologies. Innovation ecosystems are thriving, fueled by academic-industry collaborations aimed at refining CTC detection sensitivity and specificity. Regulatory frameworks, such as FDA approvals and CE marking, are becoming crucial gating factors, influencing market entry and product commercialization. The threat of substitute products, while growing, remains limited due to the unique insights CTCs offer into tumor biology and metastasis. End-user trends are increasingly focused on non-invasive diagnostics and theranostics, driving demand for advanced CTC platforms. Mergers and acquisitions (M&A) activity is a significant driver of market consolidation and technology diffusion. For instance, Precision for Medicine's acquisition of ApoCell highlights the strategic importance of acquiring robust CTC capture capabilities. We anticipate an average of 5-7 significant M&A deals annually throughout the forecast period, significantly impacting market share distribution and innovation pathways. Companies like Thermo Fisher Scientific and Qiagen NV are poised to expand their influence through strategic partnerships and product integration, aiming to capture a larger share of the burgeoning liquid biopsy market. The overall market share distribution is expected to see a shift, with dominant players focusing on expanding their CTC-based diagnostics portfolios.
Circulating Tumor Cells Industry Industry Insights & Trends
The Circulating Tumor Cells (CTC) industry is poised for substantial growth, projected to reach approximately $5,000 Million by 2033, with a compound annual growth rate (CAGR) of around 15.5% from the base year of 2025. This remarkable expansion is primarily propelled by several key market growth drivers. The escalating global incidence of cancer, coupled with a growing awareness and demand for early cancer detection and minimally invasive diagnostic methods, is creating a robust market. Advancements in CTC enrichment and CTC detection technologies, such as improved immunocytochemical technology and sophisticated molecular (RNA)-based technology, are significantly enhancing the accuracy and efficiency of CTC analysis, enabling better cancer patient stratification and treatment response monitoring. Furthermore, the increasing adoption of personalized medicine and precision oncology strategies, where CTCs play a pivotal role in guiding therapeutic decisions and assessing drug efficacy, is a significant trend. The growing investment in cancer research and development by both public and private entities, alongside favorable reimbursement policies for liquid biopsy tests in several key regions, further bolsters market growth. Technological disruptions are continuously emerging, with a focus on developing more sensitive and cost-effective CTC platforms. Evolving consumer behaviors, particularly the preference for non-invasive diagnostic approaches to minimize patient discomfort and risk associated with traditional biopsies, are also contributing to market expansion. The integration of AI and machine learning in CTC analysis is another trend expected to revolutionize the field, offering faster and more accurate interpretation of complex CTC data. The rising prevalence of targetable mutations in various cancers and the subsequent need for targeted therapies, which can be effectively monitored using CTCs, are also significant contributing factors to the upward trajectory of the CTC market.
Key Markets & Segments Leading Circulating Tumor Cells Industry
The Circulating Tumor Cells (CTC) industry is experiencing robust growth across multiple segments, with North America emerging as the dominant region in terms of market share and innovation. The United States, in particular, leads due to its strong healthcare infrastructure, significant investment in R&D for cancer diagnostics, and a high prevalence of cancer cases.
Technology Segmentation Dominance:
CTC Enrichment Methods:
- Positive Enrichment is currently leading due to its ability to capture CTCs directly using specific biomarkers. This method offers high specificity for identifying CTCs from blood samples.
- Negative Enrichment is gaining traction as it removes background cells, increasing the purity of the CTC sample, especially for rare cell populations.
- Other Technologies, including microfluidic devices and inertial focusing, are showing immense promise and are expected to capture significant market share in the coming years, offering advantages in speed and scalability.
CTC Detection Methods:
- Immunocytochemical Technology remains a cornerstone, leveraging antibodies against tumor-specific antigens for visual identification and characterization of CTCs. Its widespread use and established protocols contribute to its market leadership.
- Molecular (RNA)-based Technology is experiencing rapid growth, offering deeper insights into gene expression and identifying CTCs based on their unique RNA profiles, even when protein expression is low or absent. This segment is critical for understanding tumor heterogeneity and resistance mechanisms.
- Other CTC Detection Methods, encompassing advanced imaging techniques and single-cell sequencing, are emerging as powerful tools for comprehensive CTC analysis and are expected to drive future market expansion.
Application Segmentation Dominance:
- RNA Profiling is a key driver, enabling the analysis of gene expression patterns within CTCs to predict treatment response and identify therapeutic targets. This segment is crucial for advancing precision oncology.
- Protein Expression analysis continues to be vital for identifying specific biomarkers and understanding tumor cell surface characteristics.
- Multiple Chromosome Abnormalities detection is becoming increasingly important for assessing tumor ploidy and identifying potential genetic drivers of disease progression.
- Cellular Communication analysis is an emerging application, focusing on understanding the complex interactions between CTCs and the tumor microenvironment, offering new avenues for therapeutic intervention.
The economic growth in North America, coupled with government initiatives promoting cancer research and the early adoption of advanced medical technologies, underpins its leadership. The presence of leading players like Thermo Fisher Scientific, Qiagen NV, and Biocept Inc. further solidifies this dominance, fostering a competitive environment that drives innovation and market penetration.
Circulating Tumor Cells Industry Product Developments
Product developments in the Circulating Tumor Cells (CTC) industry are rapidly advancing the capabilities of liquid biopsy diagnostics. Innovations are focused on enhancing the sensitivity and specificity of CTC isolation and detection technologies, enabling earlier and more accurate cancer diagnosis and monitoring. Companies are introducing novel microfluidic devices and antibody-based capture systems designed for efficient and rapid CTC enumeration and characterization. Furthermore, the integration of genomic and transcriptomic analysis directly from captured CTCs is enabling deeper insights into tumor biology, driving the development of targeted therapies and personalized treatment strategies. The development of automated platforms and AI-driven analysis tools is also enhancing throughput and reducing turnaround times, making CTC analysis more accessible and clinically relevant.
Challenges in the Circulating Tumor Cells Industry Market
Despite its immense potential, the Circulating Tumor Cells (CTC) industry faces several challenges.
- Standardization: Lack of standardized protocols for CTC isolation, enumeration, and characterization across different platforms hinders comparability and regulatory acceptance.
- Cost: The high cost associated with advanced CTC analysis platforms and reagents can be a barrier to widespread adoption, particularly in resource-limited settings.
- Clinical Validation: The need for extensive clinical validation studies to demonstrate the definitive clinical utility and impact of CTC assays on patient outcomes remains a significant hurdle.
- Reimbursement: Inconsistent and limited reimbursement policies from healthcare payers for CTC-based diagnostics can affect market penetration and physician adoption.
- Technical Sensitivity: Achieving sufficient sensitivity to reliably detect very low numbers of CTCs in early-stage cancers or during treatment remains a technical challenge.
Forces Driving Circulating Tumor Cells Industry Growth
Several key forces are propelling the growth of the Circulating Tumor Cells (CTC) industry. The increasing global burden of cancer and the rising demand for non-invasive cancer diagnostics are fundamental drivers. Technological advancements in CTC enrichment and detection methodologies, including novel microfluidic technologies and highly specific biomarkers, are significantly enhancing the precision and reliability of CTC analysis. The paradigm shift towards precision medicine and the growing emphasis on personalized cancer treatment strategies are creating a strong market need for biomarkers like CTCs that can provide real-time information about tumor evolution and treatment response. Furthermore, supportive government initiatives, increasing investments in cancer research, and the development of favorable reimbursement landscapes in key markets are all contributing to the accelerating growth of the CTC sector.
Challenges in the Circulating Tumor Cells Industry Market
The long-term growth of the Circulating Tumor Cells (CTC) industry is fueled by several critical catalysts. The continuous innovation in liquid biopsy technologies, particularly in enhancing the sensitivity of CTC detection and enabling comprehensive molecular profiling, is a primary driver. Strategic partnerships between diagnostic companies, pharmaceutical firms, and academic institutions are accelerating the translation of research findings into clinical applications. Expansion into new therapeutic areas and the development of CTC assays for a wider range of cancer types, including less common malignancies, represent significant market growth opportunities. Furthermore, the increasing integration of CTC analysis into routine clinical practice for treatment monitoring, early recurrence detection, and prognostic assessment will solidify its position as a vital tool in oncology.
Emerging Opportunities in Circulating Tumor Cells Industry
Emerging opportunities in the Circulating Tumor Cells (CTC) industry are vast and transformative. The development of ultra-sensitive platforms capable of detecting minimal residual disease (MRD) in blood holds immense promise for preventing cancer recurrence. The exploration of CTCs in understanding tumor microenvironment dynamics and identifying novel therapeutic targets represents a significant frontier. The application of CTC analysis in areas beyond traditional oncology, such as monitoring drug resistance mechanisms and predicting patient response to immunotherapy, is a rapidly growing niche. The standardization of CTC analysis through collaborative efforts and the establishment of robust clinical guidelines will further unlock new markets and applications. Moreover, the potential for integrating CTC data with other omics data (genomics, proteomics) through advanced bioinformatics will lead to more sophisticated and personalized cancer management strategies.
Leading Players in the Circulating Tumor Cells Industry Sector
- BioChain Institute Inc
- Thermo Fisher Scientific
- Precision for Medicine (Formerly ApoCell)
- Menarini Silicon Biosystems
- Aviva Biosciences
- Creatv Micro Tech Inc
- Miltenyi Biotec
- LungLife AI Inc
- Sysmex Corporation
- Qiagen NV
- Advanced Cell Diagnostics Inc
- Biocept Inc
Key Milestones in Circulating Tumor Cells Industry Industry
- July 2021: Datar Cancer Genetics reported the publication of a MedTech Innovation Briefing (MIB) from the United Kingdom's National Institute for Health and Care Excellence (NICE) on its CE-marked 'Trueblood-Prostate' test, designed for precision triaging of patients to avoid unnecessary invasive biopsies. This development underscored the growing clinical utility and regulatory acceptance of CTC-based diagnostics for prostate cancer management.
- February 2021: Menarini Silicon Biosystems launched its CellMag product line, facilitating the manual enrichment and staining of rare circulating tumor cells (CTCs). This launch provided researchers and clinicians with a more accessible and flexible tool for CTC analysis, contributing to the expansion of research into various cancer types.
Strategic Outlook for Circulating Tumor Cells Industry Market
The strategic outlook for the Circulating Tumor Cells (CTC) industry is exceptionally positive, driven by a confluence of technological innovation, increasing clinical validation, and a growing demand for personalized cancer care. Key growth accelerators include the continued development of highly sensitive and multiplexed CTC detection platforms, enabling comprehensive analysis of tumor heterogeneity and evolution. Strategic collaborations between diagnostic developers and pharmaceutical companies will be crucial for integrating CTC insights into drug development pipelines and clinical trial design. The expansion of CTC applications beyond diagnosis to encompass treatment monitoring, MRD detection, and recurrence prediction will solidify its value proposition. Furthermore, ongoing efforts to standardize assays and secure robust reimbursement policies will pave the way for widespread clinical adoption, positioning the CTC industry as an indispensable component of future oncology practice.
Circulating Tumor Cells Industry Segmentation
-
1. Technology
-
1.1. CTC Enrichment Methods
- 1.1.1. Positive Enrichment
- 1.1.2. Negative Enrichment
- 1.1.3. Other Technologies
-
1.2. CTC Detection Methods
- 1.2.1. Immunocytochemical Technology
- 1.2.2. Molecular (RNA)-based Technology
- 1.2.3. Other CTC Detection Methods
-
1.1. CTC Enrichment Methods
-
2. Application
- 2.1. Multiple Chromosome Abnormalities
- 2.2. RNA Profiling
- 2.3. Protein Expression
- 2.4. Cellular Communication
- 2.5. Other Applications
Circulating Tumor Cells Industry Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. Europe
- 2.1. Germany
- 2.2. United Kingdom
- 2.3. France
- 2.4. Italy
- 2.5. Spain
- 2.6. Rest of Europe
-
3. Asia Pacific
- 3.1. China
- 3.2. Japan
- 3.3. India
- 3.4. Australia
- 3.5. South Korea
- 3.6. Rest of Asia Pacific
-
4. Middle East and Africa
- 4.1. GCC
- 4.2. South Africa
- 4.3. Rest of Middle East and Africa
-
5. South America
- 5.1. Brazil
- 5.2. Argentina
- 5.3. Rest of South America
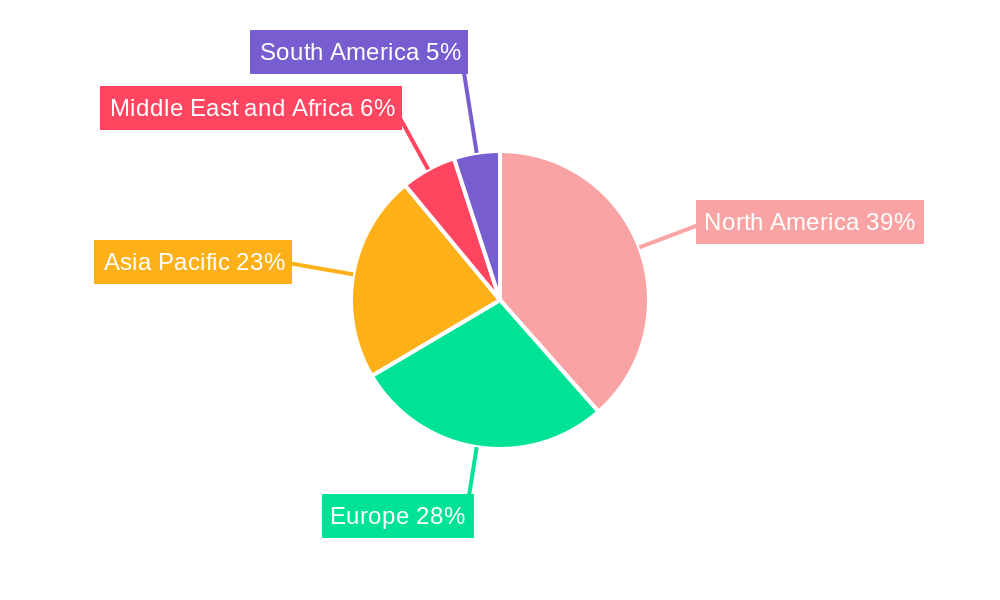
Circulating Tumor Cells Industry Regional Market Share

Geographic Coverage of Circulating Tumor Cells Industry
Circulating Tumor Cells Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 13.92% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Advancements in Biomedical Imaging and Bioengineering Technology; Rising Demand for Preventive Medicine and Companion Diagnostics; Increasing Prevalence of Cancer
- 3.3. Market Restrains
- 3.3.1. Technical Difficulties in Detection and Characterization of CTCs Associated with High Cost of Diagnosis; Lack of Awarness and Unwillingness for the Adoption of Advanced CTC Technologies
- 3.4. Market Trends
- 3.4.1. The Negative Enrichment Segment is Expected to Hold a Major Market Share in the Circulating Tumor Cells (CTC) Market
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Circulating Tumor Cells Industry Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Technology
- 5.1.1. CTC Enrichment Methods
- 5.1.1.1. Positive Enrichment
- 5.1.1.2. Negative Enrichment
- 5.1.1.3. Other Technologies
- 5.1.2. CTC Detection Methods
- 5.1.2.1. Immunocytochemical Technology
- 5.1.2.2. Molecular (RNA)-based Technology
- 5.1.2.3. Other CTC Detection Methods
- 5.1.1. CTC Enrichment Methods
- 5.2. Market Analysis, Insights and Forecast - by Application
- 5.2.1. Multiple Chromosome Abnormalities
- 5.2.2. RNA Profiling
- 5.2.3. Protein Expression
- 5.2.4. Cellular Communication
- 5.2.5. Other Applications
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. Europe
- 5.3.3. Asia Pacific
- 5.3.4. Middle East and Africa
- 5.3.5. South America
- 5.1. Market Analysis, Insights and Forecast - by Technology
- 6. North America Circulating Tumor Cells Industry Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Technology
- 6.1.1. CTC Enrichment Methods
- 6.1.1.1. Positive Enrichment
- 6.1.1.2. Negative Enrichment
- 6.1.1.3. Other Technologies
- 6.1.2. CTC Detection Methods
- 6.1.2.1. Immunocytochemical Technology
- 6.1.2.2. Molecular (RNA)-based Technology
- 6.1.2.3. Other CTC Detection Methods
- 6.1.1. CTC Enrichment Methods
- 6.2. Market Analysis, Insights and Forecast - by Application
- 6.2.1. Multiple Chromosome Abnormalities
- 6.2.2. RNA Profiling
- 6.2.3. Protein Expression
- 6.2.4. Cellular Communication
- 6.2.5. Other Applications
- 6.1. Market Analysis, Insights and Forecast - by Technology
- 7. Europe Circulating Tumor Cells Industry Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Technology
- 7.1.1. CTC Enrichment Methods
- 7.1.1.1. Positive Enrichment
- 7.1.1.2. Negative Enrichment
- 7.1.1.3. Other Technologies
- 7.1.2. CTC Detection Methods
- 7.1.2.1. Immunocytochemical Technology
- 7.1.2.2. Molecular (RNA)-based Technology
- 7.1.2.3. Other CTC Detection Methods
- 7.1.1. CTC Enrichment Methods
- 7.2. Market Analysis, Insights and Forecast - by Application
- 7.2.1. Multiple Chromosome Abnormalities
- 7.2.2. RNA Profiling
- 7.2.3. Protein Expression
- 7.2.4. Cellular Communication
- 7.2.5. Other Applications
- 7.1. Market Analysis, Insights and Forecast - by Technology
- 8. Asia Pacific Circulating Tumor Cells Industry Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Technology
- 8.1.1. CTC Enrichment Methods
- 8.1.1.1. Positive Enrichment
- 8.1.1.2. Negative Enrichment
- 8.1.1.3. Other Technologies
- 8.1.2. CTC Detection Methods
- 8.1.2.1. Immunocytochemical Technology
- 8.1.2.2. Molecular (RNA)-based Technology
- 8.1.2.3. Other CTC Detection Methods
- 8.1.1. CTC Enrichment Methods
- 8.2. Market Analysis, Insights and Forecast - by Application
- 8.2.1. Multiple Chromosome Abnormalities
- 8.2.2. RNA Profiling
- 8.2.3. Protein Expression
- 8.2.4. Cellular Communication
- 8.2.5. Other Applications
- 8.1. Market Analysis, Insights and Forecast - by Technology
- 9. Middle East and Africa Circulating Tumor Cells Industry Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Technology
- 9.1.1. CTC Enrichment Methods
- 9.1.1.1. Positive Enrichment
- 9.1.1.2. Negative Enrichment
- 9.1.1.3. Other Technologies
- 9.1.2. CTC Detection Methods
- 9.1.2.1. Immunocytochemical Technology
- 9.1.2.2. Molecular (RNA)-based Technology
- 9.1.2.3. Other CTC Detection Methods
- 9.1.1. CTC Enrichment Methods
- 9.2. Market Analysis, Insights and Forecast - by Application
- 9.2.1. Multiple Chromosome Abnormalities
- 9.2.2. RNA Profiling
- 9.2.3. Protein Expression
- 9.2.4. Cellular Communication
- 9.2.5. Other Applications
- 9.1. Market Analysis, Insights and Forecast - by Technology
- 10. South America Circulating Tumor Cells Industry Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Technology
- 10.1.1. CTC Enrichment Methods
- 10.1.1.1. Positive Enrichment
- 10.1.1.2. Negative Enrichment
- 10.1.1.3. Other Technologies
- 10.1.2. CTC Detection Methods
- 10.1.2.1. Immunocytochemical Technology
- 10.1.2.2. Molecular (RNA)-based Technology
- 10.1.2.3. Other CTC Detection Methods
- 10.1.1. CTC Enrichment Methods
- 10.2. Market Analysis, Insights and Forecast - by Application
- 10.2.1. Multiple Chromosome Abnormalities
- 10.2.2. RNA Profiling
- 10.2.3. Protein Expression
- 10.2.4. Cellular Communication
- 10.2.5. Other Applications
- 10.1. Market Analysis, Insights and Forecast - by Technology
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2025
- 11.2. Company Profiles
- 11.2.1 BioChain Institute Inc
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Thermofisher
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Precision for Medicine (Formerly ApoCell)
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Menarini Silicon Biosystems
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Aviva Biosciences
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Creatv Micro Tech Inc
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Miltenyi Biotec
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 LungLife AI Inc
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Sysmex Corporation
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Qiagen NV
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Advanced Cell Diagnostics Inc
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 Biocept Inc
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.1 BioChain Institute Inc
List of Figures
- Figure 1: Global Circulating Tumor Cells Industry Revenue Breakdown (billion, %) by Region 2025 & 2033
- Figure 2: Global Circulating Tumor Cells Industry Volume Breakdown (K Unit, %) by Region 2025 & 2033
- Figure 3: North America Circulating Tumor Cells Industry Revenue (billion), by Technology 2025 & 2033
- Figure 4: North America Circulating Tumor Cells Industry Volume (K Unit), by Technology 2025 & 2033
- Figure 5: North America Circulating Tumor Cells Industry Revenue Share (%), by Technology 2025 & 2033
- Figure 6: North America Circulating Tumor Cells Industry Volume Share (%), by Technology 2025 & 2033
- Figure 7: North America Circulating Tumor Cells Industry Revenue (billion), by Application 2025 & 2033
- Figure 8: North America Circulating Tumor Cells Industry Volume (K Unit), by Application 2025 & 2033
- Figure 9: North America Circulating Tumor Cells Industry Revenue Share (%), by Application 2025 & 2033
- Figure 10: North America Circulating Tumor Cells Industry Volume Share (%), by Application 2025 & 2033
- Figure 11: North America Circulating Tumor Cells Industry Revenue (billion), by Country 2025 & 2033
- Figure 12: North America Circulating Tumor Cells Industry Volume (K Unit), by Country 2025 & 2033
- Figure 13: North America Circulating Tumor Cells Industry Revenue Share (%), by Country 2025 & 2033
- Figure 14: North America Circulating Tumor Cells Industry Volume Share (%), by Country 2025 & 2033
- Figure 15: Europe Circulating Tumor Cells Industry Revenue (billion), by Technology 2025 & 2033
- Figure 16: Europe Circulating Tumor Cells Industry Volume (K Unit), by Technology 2025 & 2033
- Figure 17: Europe Circulating Tumor Cells Industry Revenue Share (%), by Technology 2025 & 2033
- Figure 18: Europe Circulating Tumor Cells Industry Volume Share (%), by Technology 2025 & 2033
- Figure 19: Europe Circulating Tumor Cells Industry Revenue (billion), by Application 2025 & 2033
- Figure 20: Europe Circulating Tumor Cells Industry Volume (K Unit), by Application 2025 & 2033
- Figure 21: Europe Circulating Tumor Cells Industry Revenue Share (%), by Application 2025 & 2033
- Figure 22: Europe Circulating Tumor Cells Industry Volume Share (%), by Application 2025 & 2033
- Figure 23: Europe Circulating Tumor Cells Industry Revenue (billion), by Country 2025 & 2033
- Figure 24: Europe Circulating Tumor Cells Industry Volume (K Unit), by Country 2025 & 2033
- Figure 25: Europe Circulating Tumor Cells Industry Revenue Share (%), by Country 2025 & 2033
- Figure 26: Europe Circulating Tumor Cells Industry Volume Share (%), by Country 2025 & 2033
- Figure 27: Asia Pacific Circulating Tumor Cells Industry Revenue (billion), by Technology 2025 & 2033
- Figure 28: Asia Pacific Circulating Tumor Cells Industry Volume (K Unit), by Technology 2025 & 2033
- Figure 29: Asia Pacific Circulating Tumor Cells Industry Revenue Share (%), by Technology 2025 & 2033
- Figure 30: Asia Pacific Circulating Tumor Cells Industry Volume Share (%), by Technology 2025 & 2033
- Figure 31: Asia Pacific Circulating Tumor Cells Industry Revenue (billion), by Application 2025 & 2033
- Figure 32: Asia Pacific Circulating Tumor Cells Industry Volume (K Unit), by Application 2025 & 2033
- Figure 33: Asia Pacific Circulating Tumor Cells Industry Revenue Share (%), by Application 2025 & 2033
- Figure 34: Asia Pacific Circulating Tumor Cells Industry Volume Share (%), by Application 2025 & 2033
- Figure 35: Asia Pacific Circulating Tumor Cells Industry Revenue (billion), by Country 2025 & 2033
- Figure 36: Asia Pacific Circulating Tumor Cells Industry Volume (K Unit), by Country 2025 & 2033
- Figure 37: Asia Pacific Circulating Tumor Cells Industry Revenue Share (%), by Country 2025 & 2033
- Figure 38: Asia Pacific Circulating Tumor Cells Industry Volume Share (%), by Country 2025 & 2033
- Figure 39: Middle East and Africa Circulating Tumor Cells Industry Revenue (billion), by Technology 2025 & 2033
- Figure 40: Middle East and Africa Circulating Tumor Cells Industry Volume (K Unit), by Technology 2025 & 2033
- Figure 41: Middle East and Africa Circulating Tumor Cells Industry Revenue Share (%), by Technology 2025 & 2033
- Figure 42: Middle East and Africa Circulating Tumor Cells Industry Volume Share (%), by Technology 2025 & 2033
- Figure 43: Middle East and Africa Circulating Tumor Cells Industry Revenue (billion), by Application 2025 & 2033
- Figure 44: Middle East and Africa Circulating Tumor Cells Industry Volume (K Unit), by Application 2025 & 2033
- Figure 45: Middle East and Africa Circulating Tumor Cells Industry Revenue Share (%), by Application 2025 & 2033
- Figure 46: Middle East and Africa Circulating Tumor Cells Industry Volume Share (%), by Application 2025 & 2033
- Figure 47: Middle East and Africa Circulating Tumor Cells Industry Revenue (billion), by Country 2025 & 2033
- Figure 48: Middle East and Africa Circulating Tumor Cells Industry Volume (K Unit), by Country 2025 & 2033
- Figure 49: Middle East and Africa Circulating Tumor Cells Industry Revenue Share (%), by Country 2025 & 2033
- Figure 50: Middle East and Africa Circulating Tumor Cells Industry Volume Share (%), by Country 2025 & 2033
- Figure 51: South America Circulating Tumor Cells Industry Revenue (billion), by Technology 2025 & 2033
- Figure 52: South America Circulating Tumor Cells Industry Volume (K Unit), by Technology 2025 & 2033
- Figure 53: South America Circulating Tumor Cells Industry Revenue Share (%), by Technology 2025 & 2033
- Figure 54: South America Circulating Tumor Cells Industry Volume Share (%), by Technology 2025 & 2033
- Figure 55: South America Circulating Tumor Cells Industry Revenue (billion), by Application 2025 & 2033
- Figure 56: South America Circulating Tumor Cells Industry Volume (K Unit), by Application 2025 & 2033
- Figure 57: South America Circulating Tumor Cells Industry Revenue Share (%), by Application 2025 & 2033
- Figure 58: South America Circulating Tumor Cells Industry Volume Share (%), by Application 2025 & 2033
- Figure 59: South America Circulating Tumor Cells Industry Revenue (billion), by Country 2025 & 2033
- Figure 60: South America Circulating Tumor Cells Industry Volume (K Unit), by Country 2025 & 2033
- Figure 61: South America Circulating Tumor Cells Industry Revenue Share (%), by Country 2025 & 2033
- Figure 62: South America Circulating Tumor Cells Industry Volume Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Technology 2020 & 2033
- Table 2: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Technology 2020 & 2033
- Table 3: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Application 2020 & 2033
- Table 4: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Application 2020 & 2033
- Table 5: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Region 2020 & 2033
- Table 6: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Region 2020 & 2033
- Table 7: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Technology 2020 & 2033
- Table 8: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Technology 2020 & 2033
- Table 9: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Application 2020 & 2033
- Table 10: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Application 2020 & 2033
- Table 11: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Country 2020 & 2033
- Table 12: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 13: United States Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 14: United States Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 15: Canada Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 16: Canada Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 17: Mexico Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 18: Mexico Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 19: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Technology 2020 & 2033
- Table 20: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Technology 2020 & 2033
- Table 21: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Application 2020 & 2033
- Table 22: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Application 2020 & 2033
- Table 23: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Country 2020 & 2033
- Table 24: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 25: Germany Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 26: Germany Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 27: United Kingdom Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 28: United Kingdom Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 29: France Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 30: France Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 31: Italy Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 32: Italy Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 33: Spain Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 34: Spain Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 35: Rest of Europe Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 36: Rest of Europe Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 37: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Technology 2020 & 2033
- Table 38: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Technology 2020 & 2033
- Table 39: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Application 2020 & 2033
- Table 40: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Application 2020 & 2033
- Table 41: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Country 2020 & 2033
- Table 42: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 43: China Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 44: China Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 45: Japan Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 46: Japan Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 47: India Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 48: India Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 49: Australia Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 50: Australia Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 51: South Korea Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 52: South Korea Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 53: Rest of Asia Pacific Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 54: Rest of Asia Pacific Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 55: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Technology 2020 & 2033
- Table 56: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Technology 2020 & 2033
- Table 57: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Application 2020 & 2033
- Table 58: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Application 2020 & 2033
- Table 59: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Country 2020 & 2033
- Table 60: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 61: GCC Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 62: GCC Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 63: South Africa Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 64: South Africa Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 65: Rest of Middle East and Africa Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 66: Rest of Middle East and Africa Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 67: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Technology 2020 & 2033
- Table 68: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Technology 2020 & 2033
- Table 69: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Application 2020 & 2033
- Table 70: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Application 2020 & 2033
- Table 71: Global Circulating Tumor Cells Industry Revenue billion Forecast, by Country 2020 & 2033
- Table 72: Global Circulating Tumor Cells Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 73: Brazil Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 74: Brazil Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 75: Argentina Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 76: Argentina Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 77: Rest of South America Circulating Tumor Cells Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 78: Rest of South America Circulating Tumor Cells Industry Volume (K Unit) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Circulating Tumor Cells Industry?
The projected CAGR is approximately 13.92%.
2. Which companies are prominent players in the Circulating Tumor Cells Industry?
Key companies in the market include BioChain Institute Inc, Thermofisher, Precision for Medicine (Formerly ApoCell), Menarini Silicon Biosystems, Aviva Biosciences, Creatv Micro Tech Inc, Miltenyi Biotec, LungLife AI Inc, Sysmex Corporation, Qiagen NV, Advanced Cell Diagnostics Inc, Biocept Inc.
3. What are the main segments of the Circulating Tumor Cells Industry?
The market segments include Technology, Application.
4. Can you provide details about the market size?
The market size is estimated to be USD 14.04 billion as of 2022.
5. What are some drivers contributing to market growth?
Advancements in Biomedical Imaging and Bioengineering Technology; Rising Demand for Preventive Medicine and Companion Diagnostics; Increasing Prevalence of Cancer.
6. What are the notable trends driving market growth?
The Negative Enrichment Segment is Expected to Hold a Major Market Share in the Circulating Tumor Cells (CTC) Market.
7. Are there any restraints impacting market growth?
Technical Difficulties in Detection and Characterization of CTCs Associated with High Cost of Diagnosis; Lack of Awarness and Unwillingness for the Adoption of Advanced CTC Technologies.
8. Can you provide examples of recent developments in the market?
In July 2021, Datar Cancer Genetics reported the publication of a MedTech Innovation Briefing (MIB) from the United Kingdom's National Institute for Health and Care Excellence (NICE) on its CE-marked 'Trueblood-Prostate' test to be used for precision triaging of patients to avoid unnecessary invasive biopsies.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in billion and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Circulating Tumor Cells Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Circulating Tumor Cells Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Circulating Tumor Cells Industry?
To stay informed about further developments, trends, and reports in the Circulating Tumor Cells Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
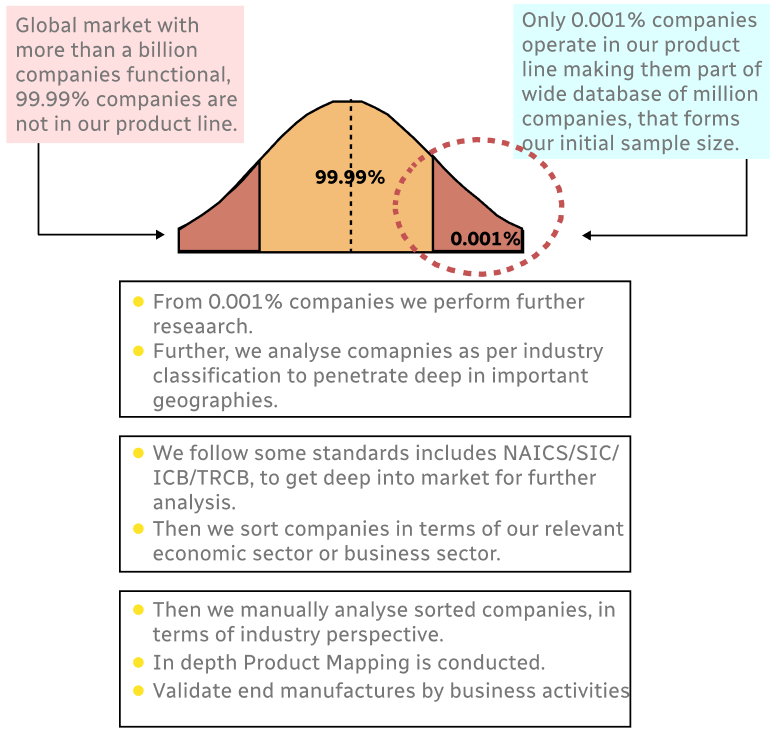
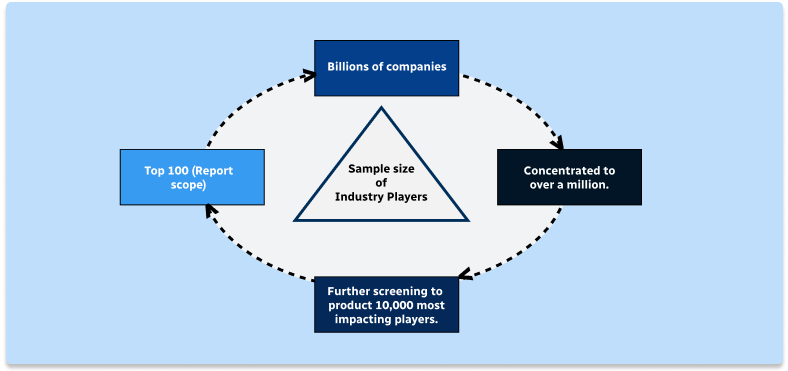
Step 1 - Identification of Relevant Samples Size from Population Database
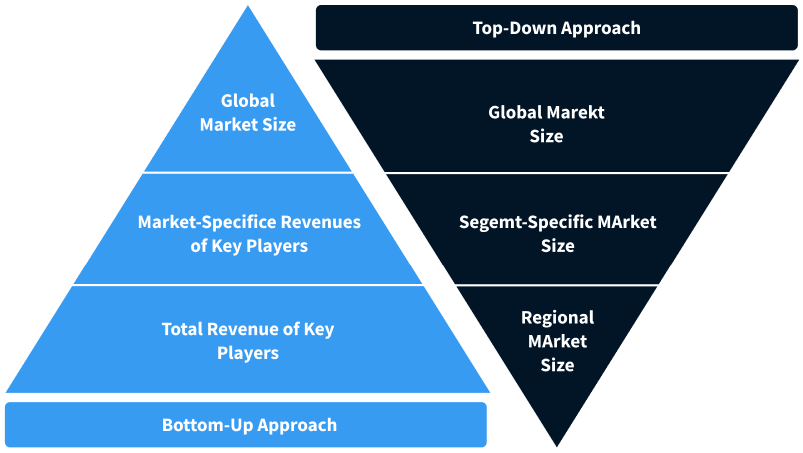
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
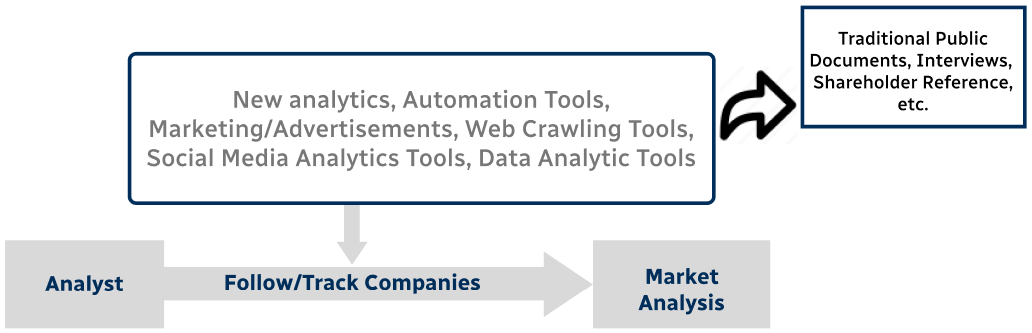
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


