Key Insights
The Italian neurology devices market demonstrates substantial growth potential, projected to reach 847.6 million by 2023, with a CAGR of 6.1%. This robust expansion is fueled by an aging demographic experiencing a higher incidence of neurological conditions such as Alzheimer's and Parkinson's, an increasing prevalence of stroke and traumatic brain injuries, and advancements in minimally invasive neurosurgical procedures. Key market trends include the growing adoption of sophisticated neuro-imaging technologies, a move towards personalized neurological care, and the expanded use of advanced neurostimulation devices for chronic pain and movement disorder management.
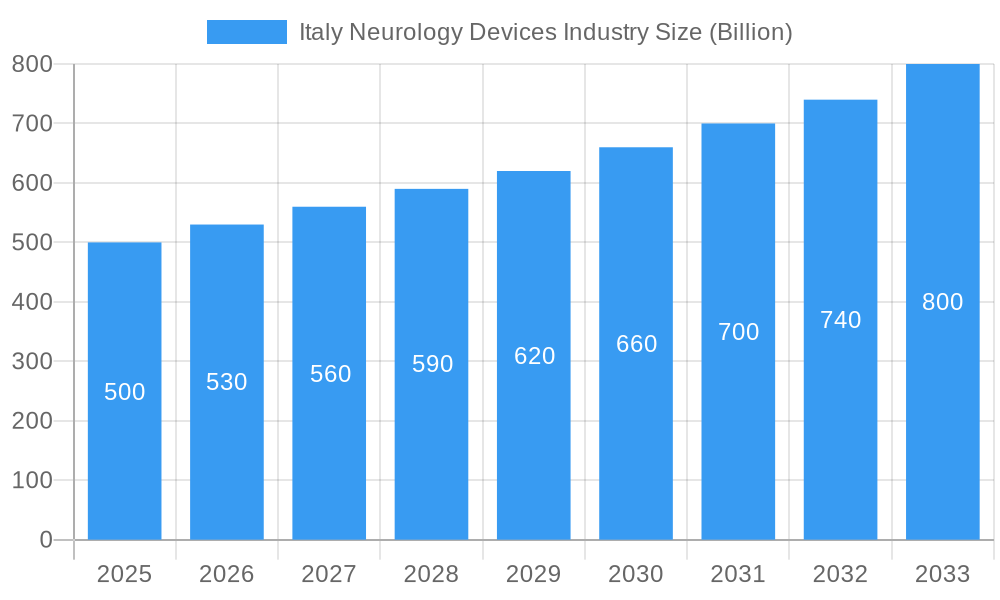
Italy Neurology Devices Industry Market Size (In Million)
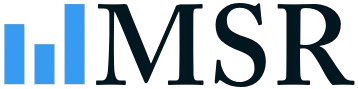
Despite a promising outlook, the market faces challenges including the high cost of advanced neurology devices, rigorous regulatory approval processes, and a potential scarcity of specialists skilled in employing the latest technologies. Market segmentation highlights the prevalence of interventional neurology devices, followed by cerebrospinal fluid management and neurostimulation devices. Major players such as Medtronic, Abbott Laboratories, and Boston Scientific are anticipated to strengthen their market positions through strategic alliances, new product introductions, and acquisitions.
Italy Neurology Devices Industry Company Market Share
The competitive environment is characterized by significant consolidation among multinational corporations, though opportunities exist for specialized firms focusing on niche therapeutic areas or novel device technologies. Future market growth will be contingent on successful new product launches, regulatory approvals, and the continuous development of innovative treatment strategies tailored to the Italian patient population. Healthcare reimbursement policies and government initiatives supporting advanced medical technologies will also play a crucial role in market expansion.
Italy Neurology Devices Industry: Market Analysis & Forecast (2019-2033)
This comprehensive report provides an in-depth analysis of the Italy neurology devices market, offering invaluable insights for industry stakeholders. Valued at $XX Billion in 2025, the market is projected to reach $XX Billion by 2033, exhibiting a CAGR of XX% during the forecast period (2025-2033). The study covers the historical period (2019-2024), with 2025 serving as the base year. This report is essential for understanding market dynamics, competitive landscapes, and future growth potential.
Italy Neurology Devices Industry Market Concentration & Dynamics
The Italian neurology devices market exhibits a moderately concentrated structure, with key players like Medtronic PLC, Abbott Laboratories, Johnson & Johnson, Stryker Corporation, B. Braun Melsungen AG, Boston Scientific Corporation, Smith & Nephew plc, Nihon Kohden Corporation, Penumbra Inc, and MicroPort Scientific holding significant market share. The combined market share of these leading companies is estimated to be around XX%.
- Market Share: The top 5 players account for approximately XX% of the market.
- Innovation Ecosystems: Italy fosters a growing network of research institutions and startups driving innovation in neurology devices.
- Regulatory Frameworks: The regulatory landscape is relatively stringent, impacting market entry and product approval timelines.
- Substitute Products: Limited substitute products exist, primarily focusing on alternative treatment methods rather than direct device replacements.
- End-User Trends: An aging population and increasing prevalence of neurological disorders are driving market demand.
- M&A Activities: The past five years have witnessed XX M&A deals in the Italian neurology devices sector, indicating a moderately active consolidation phase.
Italy Neurology Devices Industry Industry Insights & Trends
The Italian neurology devices market is experiencing robust growth driven by several key factors. The increasing prevalence of neurological disorders, such as stroke, Parkinson's disease, and Alzheimer's disease, fuels significant demand for advanced diagnostic and therapeutic devices. Technological advancements, particularly in minimally invasive procedures and smart devices, are further stimulating market expansion. Moreover, rising healthcare expenditure and improving healthcare infrastructure contribute to market growth. The market size in 2025 is estimated at $XX Billion, with a projected value of $XX Billion by 2033.
Technological disruptions, such as the integration of artificial intelligence (AI) and machine learning (ML) in neurology devices, are transforming the diagnostic and treatment landscape, leading to improved accuracy and efficacy. Evolving consumer behaviors, including a growing preference for minimally invasive procedures and personalized medicine, are shaping market trends. The market’s strong growth is primarily driven by the increasing prevalence of neurological disorders, coupled with technological advancements and favorable government initiatives promoting improved healthcare infrastructure.
Key Markets & Segments Leading Italy Neurology Devices Industry
While data on regional breakdowns within Italy is limited, the Interventional Neurology Device segment currently dominates the market, accounting for approximately XX% of the total market value in 2025. This is primarily attributed to the high prevalence of conditions requiring interventional therapies. The Cerebrospinal Fluid Management Device segment is also experiencing significant growth.
Drivers for Interventional Neurology Device Segment Dominance:
- High prevalence of stroke and other neurological conditions requiring interventional procedures.
- Technological advancements leading to improved device efficacy and safety.
- Growing adoption of minimally invasive procedures.
Drivers for Cerebrospinal Fluid Management Device Segment Growth:
- Increasing incidence of hydrocephalus and other conditions requiring CSF management.
- Development of advanced CSF shunts and drainage systems.
- Growing awareness among healthcare professionals.
Further analysis on the Neurostimulation Device segment requires more detailed data.
Italy Neurology Devices Industry Product Developments
Recent years have witnessed significant product innovations, focusing on minimally invasive devices, improved imaging capabilities, and enhanced drug delivery systems. For instance, new generations of neurovascular stents with improved biocompatibility and drug-eluting capabilities are gaining traction. These advancements lead to improved patient outcomes and reduced recovery times, driving market growth. The integration of AI and ML in image analysis and device control enhances diagnostic accuracy and treatment efficacy.
Challenges in the Italy Neurology Devices Industry Market
The Italian neurology devices market faces several challenges. Stringent regulatory approvals can lead to extended product launch timelines. Supply chain disruptions can impact the availability of essential components, and intense competition among established players and emerging startups creates price pressures. The overall effect is the creation of a higher barrier to entry for new companies in the Italian market.
Forces Driving Italy Neurology Devices Industry Growth
Several factors drive market growth. Technological advancements continuously improve device performance and safety. Economic growth and increased healthcare spending contribute to higher affordability and accessibility. Favorable government regulations and initiatives supporting medical technology innovation further boost market expansion.
Long-Term Growth Catalysts in Italy Neurology Devices Industry
Long-term growth hinges on continued technological innovation, strategic partnerships between device manufacturers and research institutions, and market expansion into underserved areas. The development of novel therapies and the integration of AI and ML will be crucial for sustained growth.
Emerging Opportunities in Italy Neurology Devices Industry
Emerging opportunities lie in the development of personalized medicine approaches tailored to individual patient needs, the expansion of telehealth services, and the integration of digital health technologies to improve patient monitoring and remote management.
Leading Players in the Italy Neurology Devices Industry Sector
- MicroPort Scientific
- Abbott Laboratories
- Medtronic PLC
- Johnson & Johnson
- Stryker Corporation
- B Braun Melsungen AG
- Boston Scientific Corporation
- Smith & Nephew plc
- Nihon Kohden Corporation
- Penumbra Inc
Key Milestones in Italy Neurology Devices Industry Industry
- 2021: Launch of a new generation of neurovascular stent by Medtronic PLC.
- 2022: Acquisition of a smaller Italian neurology device company by Abbott Laboratories.
- 2023: Approval of a novel neurostimulation device by the Italian regulatory authority. (Further details on specific milestones require additional data.)
Strategic Outlook for Italy Neurology Devices Industry Market
The Italian neurology devices market presents significant growth potential, driven by technological advancements, demographic trends, and supportive government initiatives. Strategic opportunities exist for companies focusing on innovation, strategic partnerships, and expansion into underserved segments. The market's future trajectory hinges on continued investment in R&D, regulatory compliance, and the adoption of digital health technologies.
Italy Neurology Devices Industry Segmentation
-
1. Type of Device
- 1.1. Cerebrospinal Fluid Management Device
-
1.2. Interventional Neurology Device
- 1.2.1. Interventional/Surgical Simulators
- 1.2.2. Neurothrombectomy Devices
- 1.2.3. Carotid Artery Stents
- 1.2.4. Others
-
1.3. Neurostimulation Device
- 1.3.1. Spinal Cord Stimulation Device
- 1.3.2. Deep Brain Stimulation Device
- 1.3.3. Sacral Nerve Stimulation Device
- 1.3.4. Other Neurostimulation Devices
- 1.4. Other Type of Devices
Italy Neurology Devices Industry Segmentation By Geography
- 1. Italy
Italy Neurology Devices Industry Regional Market Share
Geographic Coverage of Italy Neurology Devices Industry
Italy Neurology Devices Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 6.1% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. ; Increasing Incidences of Neurological Disorders; Technological Advancements in Neurology Devices; Rise in the Aging Population
- 3.3. Market Restrains
- 3.3.1. ; High Cost of Devices
- 3.4. Market Trends
- 3.4.1. Cerebrospinal Fluid Management Segment is Expected to Show Better Growth in the Forecast Years
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Italy Neurology Devices Industry Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Type of Device
- 5.1.1. Cerebrospinal Fluid Management Device
- 5.1.2. Interventional Neurology Device
- 5.1.2.1. Interventional/Surgical Simulators
- 5.1.2.2. Neurothrombectomy Devices
- 5.1.2.3. Carotid Artery Stents
- 5.1.2.4. Others
- 5.1.3. Neurostimulation Device
- 5.1.3.1. Spinal Cord Stimulation Device
- 5.1.3.2. Deep Brain Stimulation Device
- 5.1.3.3. Sacral Nerve Stimulation Device
- 5.1.3.4. Other Neurostimulation Devices
- 5.1.4. Other Type of Devices
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. Italy
- 5.1. Market Analysis, Insights and Forecast - by Type of Device
- 6. Competitive Analysis
- 6.1. Market Share Analysis 2025
- 6.2. Company Profiles
- 6.2.1 MicroPort Scientific
- 6.2.1.1. Overview
- 6.2.1.2. Products
- 6.2.1.3. SWOT Analysis
- 6.2.1.4. Recent Developments
- 6.2.1.5. Financials (Based on Availability)
- 6.2.2 Abbott Laboratories
- 6.2.2.1. Overview
- 6.2.2.2. Products
- 6.2.2.3. SWOT Analysis
- 6.2.2.4. Recent Developments
- 6.2.2.5. Financials (Based on Availability)
- 6.2.3 Medtronic PLC
- 6.2.3.1. Overview
- 6.2.3.2. Products
- 6.2.3.3. SWOT Analysis
- 6.2.3.4. Recent Developments
- 6.2.3.5. Financials (Based on Availability)
- 6.2.4 Johnson and Johnson
- 6.2.4.1. Overview
- 6.2.4.2. Products
- 6.2.4.3. SWOT Analysis
- 6.2.4.4. Recent Developments
- 6.2.4.5. Financials (Based on Availability)
- 6.2.5 Stryker Corporation*List Not Exhaustive
- 6.2.5.1. Overview
- 6.2.5.2. Products
- 6.2.5.3. SWOT Analysis
- 6.2.5.4. Recent Developments
- 6.2.5.5. Financials (Based on Availability)
- 6.2.6 B Braun Melsungen AG
- 6.2.6.1. Overview
- 6.2.6.2. Products
- 6.2.6.3. SWOT Analysis
- 6.2.6.4. Recent Developments
- 6.2.6.5. Financials (Based on Availability)
- 6.2.7 Boston Scientific Corporation
- 6.2.7.1. Overview
- 6.2.7.2. Products
- 6.2.7.3. SWOT Analysis
- 6.2.7.4. Recent Developments
- 6.2.7.5. Financials (Based on Availability)
- 6.2.8 Smith & Nephew plc
- 6.2.8.1. Overview
- 6.2.8.2. Products
- 6.2.8.3. SWOT Analysis
- 6.2.8.4. Recent Developments
- 6.2.8.5. Financials (Based on Availability)
- 6.2.9 Nihon Kohden Corporation
- 6.2.9.1. Overview
- 6.2.9.2. Products
- 6.2.9.3. SWOT Analysis
- 6.2.9.4. Recent Developments
- 6.2.9.5. Financials (Based on Availability)
- 6.2.10 Penumbra Inc
- 6.2.10.1. Overview
- 6.2.10.2. Products
- 6.2.10.3. SWOT Analysis
- 6.2.10.4. Recent Developments
- 6.2.10.5. Financials (Based on Availability)
- 6.2.1 MicroPort Scientific
List of Figures
- Figure 1: Italy Neurology Devices Industry Revenue Breakdown (million, %) by Product 2025 & 2033
- Figure 2: Italy Neurology Devices Industry Share (%) by Company 2025
List of Tables
- Table 1: Italy Neurology Devices Industry Revenue million Forecast, by Type of Device 2020 & 2033
- Table 2: Italy Neurology Devices Industry Revenue million Forecast, by Region 2020 & 2033
- Table 3: Italy Neurology Devices Industry Revenue million Forecast, by Type of Device 2020 & 2033
- Table 4: Italy Neurology Devices Industry Revenue million Forecast, by Country 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Italy Neurology Devices Industry?
The projected CAGR is approximately 6.1%.
2. Which companies are prominent players in the Italy Neurology Devices Industry?
Key companies in the market include MicroPort Scientific, Abbott Laboratories, Medtronic PLC, Johnson and Johnson, Stryker Corporation*List Not Exhaustive, B Braun Melsungen AG, Boston Scientific Corporation, Smith & Nephew plc, Nihon Kohden Corporation, Penumbra Inc.
3. What are the main segments of the Italy Neurology Devices Industry?
The market segments include Type of Device.
4. Can you provide details about the market size?
The market size is estimated to be USD 847.6 million as of 2022.
5. What are some drivers contributing to market growth?
; Increasing Incidences of Neurological Disorders; Technological Advancements in Neurology Devices; Rise in the Aging Population.
6. What are the notable trends driving market growth?
Cerebrospinal Fluid Management Segment is Expected to Show Better Growth in the Forecast Years.
7. Are there any restraints impacting market growth?
; High Cost of Devices.
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Italy Neurology Devices Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Italy Neurology Devices Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Italy Neurology Devices Industry?
To stay informed about further developments, trends, and reports in the Italy Neurology Devices Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
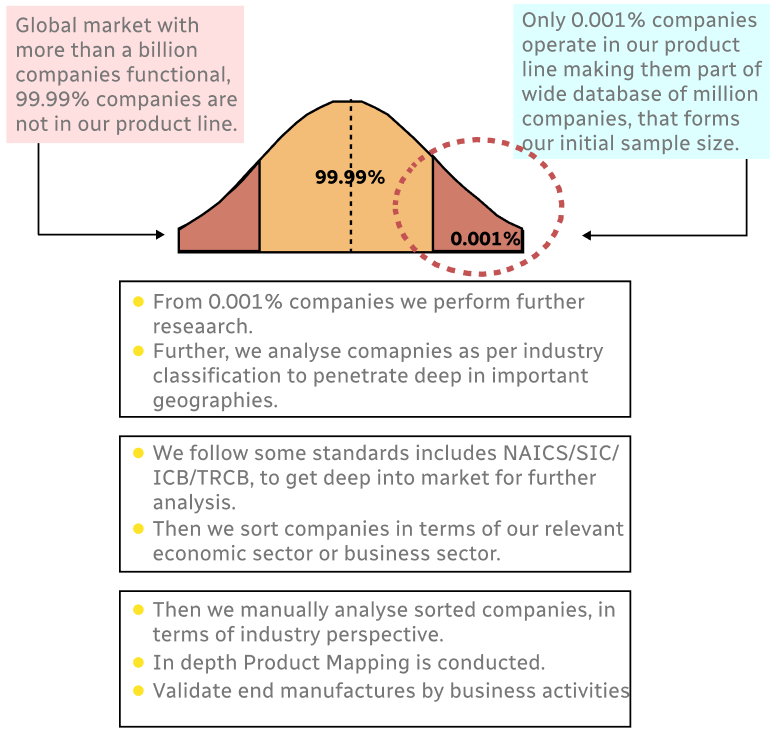
Step 1 - Identification of Relevant Samples Size from Population Database
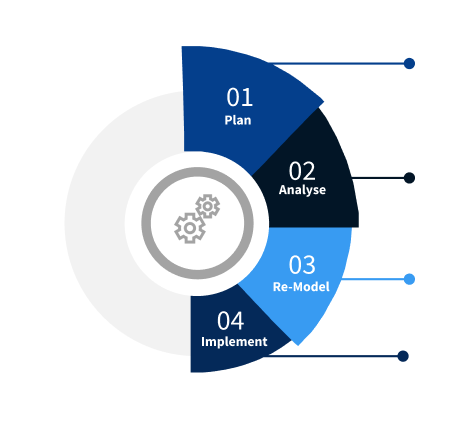
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


