Key Insights
The North America Autoimmune Disease Diagnostics Market is poised for significant expansion, driven by an increasing prevalence of autoimmune disorders and advancements in diagnostic technologies. The market is projected to reach a valuation of 2.05 Billion by 2025, demonstrating a robust Compound Annual Growth Rate (CAGR) of 5.53% through 2033. This growth is underpinned by heightened awareness among healthcare professionals and patients regarding the early detection and management of these complex conditions. Key market drivers include the rising incidence of conditions like Rheumatoid Arthritis, Psoriasis, and Multiple Sclerosis, coupled with the growing demand for personalized medicine approaches. Furthermore, continuous innovation in diagnostic assays, including sophisticated immunologic and antibody tests, is expanding the diagnostic capabilities and contributing to market buoyancy. The increasing adoption of regular laboratory tests and inflammatory marker assessments also plays a crucial role in identifying potential autoimmune issues at earlier stages.
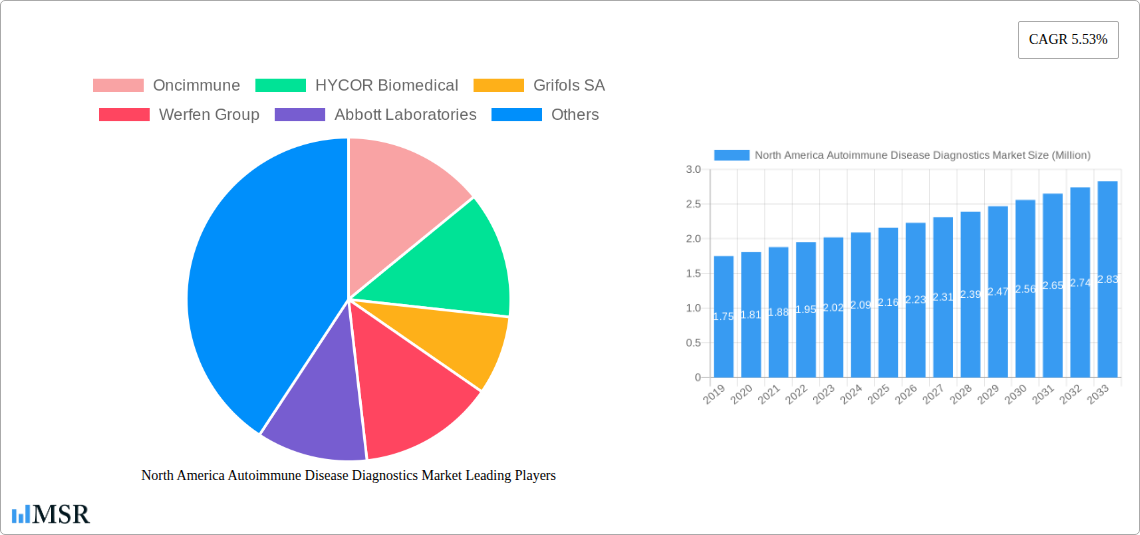
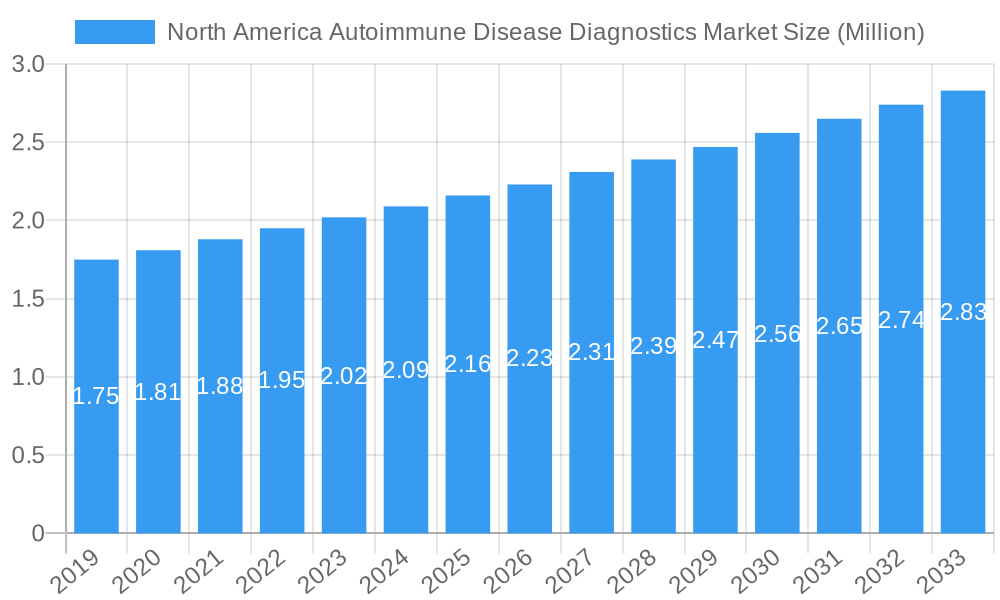
North America Autoimmune Disease Diagnostics Market Market Size (In Million)

The market's trajectory is further shaped by evolving trends such as the development of novel biomarkers and the integration of artificial intelligence in diagnostic interpretation, promising greater accuracy and efficiency. While the market exhibits strong growth potential, certain restraints, such as the high cost of advanced diagnostic equipment and limited reimbursement policies in some regions, may present challenges. However, the expanding healthcare infrastructure and a growing focus on proactive disease management across the United States, Canada, and Mexico are expected to mitigate these restraints. The market segmentation reveals a strong emphasis on disease types, with Systemic Autoimmune Diseases commanding a significant share, and diagnostic tests like immunologic assays and antibody tests being central to market activity. Leading companies are actively investing in research and development to introduce innovative diagnostic solutions, ensuring the market remains dynamic and responsive to evolving healthcare needs.
North America Autoimmune Disease Diagnostics Market Company Market Share

Here's an SEO-optimized, engaging report description for the North America Autoimmune Disease Diagnostics Market, incorporating your specified details and structure:
Report Title: North America Autoimmune Disease Diagnostics Market: Size, Share, Trends, and Forecast (2019–2033) – Comprehensive Analysis of Key Players and Emerging Opportunities
Report Description:
Uncover critical insights into the rapidly expanding North America Autoimmune Disease Diagnostics Market. This in-depth report provides a comprehensive analysis of market size, growth drivers, trends, and forecasts for the period 2019–2033, with a base and estimated year of 2025. Navigate the competitive landscape, understand key market segments, and identify future opportunities within the United States, Canada, and Mexico. Our research delves into autoimmune disease diagnosis, diagnostic test advancements, and the impact of novel diagnostic technologies. Essential for diagnostic assay manufacturers, biotechnology companies, pharmaceutical firms, research institutions, and healthcare providers seeking to capitalize on this dynamic market.
North America Autoimmune Disease Diagnostics Market Market Concentration & Dynamics
The North America Autoimmune Disease Diagnostics Market exhibits a moderate to high market concentration, characterized by the presence of established global players and emerging innovators. Key companies like Abbott Laboratories, Siemens Healthineers Inc., Thermo Fisher Scientific, and F Hoffmann-la Roche hold significant market shares due to their extensive product portfolios and robust distribution networks. The innovation ecosystem is driven by continuous research and development in areas such as autoimmune disease markers, advanced immunoassay development, and personalized diagnostics. Regulatory frameworks, overseen by bodies like the U.S. Food and Drug Administration (FDA) and Health Canada, play a crucial role in shaping market entry and product approvals, ensuring the efficacy and safety of diagnostic solutions. While direct substitute products for autoimmune disease diagnostics are limited, the evolving landscape of biomarker discovery and early disease detection methods presents a dynamic competitive environment. End-user trends indicate a growing demand for minimally invasive tests, faster turnaround times, and integrated diagnostic platforms. Mergers and acquisitions (M&A) activities are moderately prevalent, with companies strategically acquiring smaller firms to enhance their technological capabilities or expand their market reach. For instance, the acquisition of key technologies or specialized diagnostic companies contributes to market consolidation and the strengthening of competitive positions. The market witnessed approximately 5-8 M&A deals annually in the recent historical period, signaling strategic consolidation.
North America Autoimmune Disease Diagnostics Market Industry Insights & Trends
The North America Autoimmune Disease Diagnostics Market is experiencing robust growth, projected to reach an estimated market size of $XX Billion in 2025, with a Compound Annual Growth Rate (CAGR) of XX% during the 2025–2033 forecast period. This expansion is fueled by several key market growth drivers, including the increasing prevalence of autoimmune disorders across the continent, a growing awareness among both healthcare professionals and patients about the importance of early and accurate diagnosis, and significant advancements in diagnostic technology. Technological disruptions are revolutionizing the field, with the adoption of next-generation sequencing (NGS), high-throughput screening, and multiplex assays enabling the simultaneous detection of multiple biomarkers and aiding in the differentiation of complex autoimmune conditions. The evolution of consumer behaviors is also a significant factor; patients are becoming more proactive in managing their health, demanding more precise diagnostic information and personalized treatment approaches. This shift is driving the development of at-home diagnostic kits and point-of-care testing solutions, enhancing accessibility and convenience. Furthermore, the expanding research into novel autoimmune disease targets and the development of companion diagnostics for targeted therapies are contributing to market expansion. The increasing investment in R&D by leading diagnostic companies is a testament to the perceived potential of this sector. The growing adoption of digital health platforms and AI-driven diagnostic tools is further streamlining the diagnostic process and improving patient outcomes.
Key Markets & Segments Leading North America Autoimmune Disease Diagnostics Market
The United States stands as the dominant geography in the North America Autoimmune Disease Diagnostics Market, driven by its advanced healthcare infrastructure, high healthcare expenditure, and a robust pipeline of R&D activities. Economic growth in the US provides substantial funding for healthcare innovations, including diagnostic advancements. The country’s extensive network of specialized clinics and research institutions facilitates the adoption of cutting-edge diagnostic technologies.
Disease Type:
- Systemic Autoimmune Disease: This segment leads due to the high prevalence and complexity of conditions like Rheumatoid Arthritis, Psoriasis, Systemic Lupus Erythematosus (SLE), and Multiple Sclerosis. The development of sophisticated diagnostic tests for these widespread diseases significantly bolsters market revenue.
- Rheumatoid Arthritis: Driven by a large patient population and the availability of sensitive antibody tests.
- Systemic Lupus Erythematosus (SLE): Benefiting from advancements in immunologic assays and the development of specific antibody detection kits.
- Multiple Sclerosis: Supported by sophisticated neuroimaging and biomarker analysis.
- Localized Autoimmune Disease: While a smaller segment, it is experiencing steady growth, particularly in areas like Inflammatory Bowel disease and Type 1 Diabetes, due to increasing diagnostic capabilities and a growing understanding of localized autoimmune pathology.
- Inflammatory Bowel Disease (IBD): Driven by advanced serological and genetic testing.
- Type 1 Diabetes: Supported by antibody testing for early diagnosis and monitoring.
Diagnostic Test:
- Antibody Tests: These are the cornerstone of autoimmune disease diagnostics, offering high specificity and sensitivity for identifying autoantibodies associated with various conditions. The continuous development of novel antibody panels and multiplex assays significantly contributes to the market's growth.
- Immunologic Assays: Including ELISA, chemiluminescence, and immunofluorescence, these remain critical for detecting specific antibodies and antigens.
- Inflammatory Markers: Essential for monitoring disease activity and response to treatment, these tests, such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), are widely utilized.
- Regular Laboratory Tests: Foundational tests that provide initial screening and support differential diagnosis.
Geography:
- United States: Dominates due to high healthcare spending, strong regulatory support for diagnostic innovation, and a large patient pool.
- Canada: Exhibits steady growth driven by an increasing focus on chronic disease management and diagnostic advancements.
- Mexico: Shows promising growth potential due to a growing healthcare sector and rising awareness of autoimmune diseases.
North America Autoimmune Disease Diagnostics Market Product Developments
Product developments in the North America Autoimmune Disease Diagnostics Market are characterized by a drive towards enhanced accuracy, speed, and comprehensiveness. Innovations focus on creating multiplex assays that can detect multiple autoantibodies simultaneously, offering a more efficient and cost-effective diagnostic pathway for complex autoimmune conditions. Advances in immunoassay platforms, including chemiluminescent and fluorescent immunoassay technologies, are providing higher sensitivity and specificity. The development of novel biomarker panels for early disease detection and prognosis is a significant trend. Furthermore, the integration of digital technologies with diagnostic devices is enabling better data management and analysis, facilitating personalized medicine approaches.
Challenges in the North America Autoimmune Disease Diagnostics Market Market
The North America Autoimmune Disease Diagnostics Market faces several challenges that impact its growth trajectory. High development costs for new diagnostic assays, coupled with stringent regulatory approval processes, can significantly lengthen time-to-market and increase investment risks. Reimbursement policies can also be a barrier, as not all advanced diagnostic tests may be adequately covered by insurance providers, potentially limiting patient access. Interoperability issues between different diagnostic platforms and electronic health records can hinder seamless data integration. Finally, a shortage of skilled laboratory professionals capable of operating and interpreting results from complex diagnostic equipment poses a constant challenge to operational efficiency.
Forces Driving North America Autoimmune Disease Diagnostics Market Growth
Several powerful forces are propelling the growth of the North America Autoimmune Disease Diagnostics Market. The increasing incidence and prevalence of autoimmune diseases across all age groups is a primary driver. Advancements in diagnostic technologies, such as highly sensitive antibody tests and immunologic assays, are enabling earlier and more accurate diagnoses. Growing patient and physician awareness of autoimmune conditions and the importance of timely intervention further fuels demand for diagnostic solutions. Supportive government initiatives and increasing investments in biotechnology research and development are also fostering innovation and market expansion. The development of specialized diagnostic tests for specific autoimmune conditions, like those for Rheumatoid Arthritis and Systemic Lupus Erythematosus (SLE), is a key growth accelerator.
Challenges in the North America Autoimmune Disease Diagnostics Market Market
Long-term growth catalysts for the North America Autoimmune Disease Diagnostics Market are deeply rooted in technological innovation and evolving healthcare paradigms. The continued exploration and validation of novel biomarkers for early and predictive diagnosis of autoimmune diseases, including genetic markers and protein biomarkers, will unlock significant potential. Partnerships between diagnostic companies and academic institutions are crucial for accelerating research and translation into clinical practice. The expansion of point-of-care diagnostics for autoimmune conditions, allowing for faster results and improved patient management in diverse settings, represents a major growth opportunity. Furthermore, the integration of artificial intelligence (AI) and machine learning (ML) in analyzing complex diagnostic data will lead to more precise diagnoses and personalized treatment strategies. The increasing focus on companion diagnostics for targeted therapies will also drive sustained market growth.
Emerging Opportunities in North America Autoimmune Disease Diagnostics Market
Emerging opportunities in the North America Autoimmune Disease Diagnostics Market are abundant, driven by unmet clinical needs and technological advancements. The development of non-invasive diagnostic methods, such as liquid biopsies, for autoimmune diseases presents a significant opportunity. The growing demand for personalized diagnostics tailored to individual patient profiles and genetic predispositions will create niche markets. Expansion into less-diagnosed autoimmune conditions and the development of diagnostic tools for rare autoimmune diseases will open new avenues. The increasing adoption of digital health platforms and telemedicine presents opportunities for remote diagnostic monitoring and consultation. Furthermore, the growing interest in predictive diagnostics to identify individuals at high risk of developing autoimmune diseases before symptom onset offers a substantial future growth area.
Leading Players in the North America Autoimmune Disease Diagnostics Market Sector
- Oncimmune
- HYCOR Biomedical
- Grifols SA
- Werfen Group
- Abbott Laboratories
- Siemens Healthineers Inc.
- Bio-rad Laboratories
- Myriad Genetics
- Euroimmun AG (Perkinelmer Inc.)
- F Hoffmann-la Roche
- Thermo Fisher Scientific
- Trinity Biotech
Key Milestones in North America Autoimmune Disease Diagnostics Market Industry
- February 2023: Edesa Biotech received approval from Health Canada for a phase II clinical trial of its EB06 monoclonal antibody candidate to treat vitiligo, a life-altering autoimmune disease, highlighting advancements in therapeutic and diagnostic research for autoimmune conditions.
- June 2022: Thermo Scientific received United States FDA clearance for the EliA RNA Pol III and EliA Rib-P tests for aiding in the diagnosis of systemic sclerosis and systemic lupus erythematosus (SLE), demonstrating continued innovation in diagnostic test development for critical autoimmune diseases.
Strategic Outlook for North America Autoimmune Disease Diagnostics Market Market
The strategic outlook for the North America Autoimmune Disease Diagnostics Market is exceptionally positive, fueled by sustained innovation and a growing understanding of autoimmune pathology. Future growth will be accelerated by the continued development of next-generation diagnostic assays that offer greater precision and speed, alongside the integration of AI and machine learning for enhanced data analysis and predictive capabilities. Strategic partnerships between diagnostic providers, pharmaceutical companies, and research institutions will be crucial for translating scientific discoveries into marketable products. The expansion of point-of-care testing and at-home diagnostic solutions will further broaden market access and patient convenience. Emphasis on personalized medicine and companion diagnostics will create lucrative opportunities for companies offering specialized diagnostic solutions. Overall, the market is poised for significant expansion as healthcare systems increasingly prioritize early detection and precise management of autoimmune diseases.
North America Autoimmune Disease Diagnostics Market Segmentation
-
1. Disease Type
-
1.1. Systemic Autoimmune Disease
- 1.1.1. Rheumatoid Arthritis
- 1.1.2. Psoriasis
- 1.1.3. Systemic Lupus Erythematosus (SLE)
- 1.1.4. Multiple Sclerosis
- 1.1.5. Other Disease Types
-
1.2. Localized Autoimmune Disease
- 1.2.1. Inflammatory Bowel disease
- 1.2.2. Type 1 Diabetes
- 1.2.3. Thyroid
- 1.2.4. Other Localized Autoimmune Diseases
-
1.1. Systemic Autoimmune Disease
-
2. Diagnostic Test
- 2.1. Regular Laboratory Tests
- 2.2. Inflammatory Markers
- 2.3. Immunologic Assays
- 2.4. Antibody Tests
- 2.5. Other Tests
-
3. Geography
- 3.1. United States
- 3.2. Canada
- 3.3. Mexico
North America Autoimmune Disease Diagnostics Market Segmentation By Geography
- 1. United States
- 2. Canada
- 3. Mexico
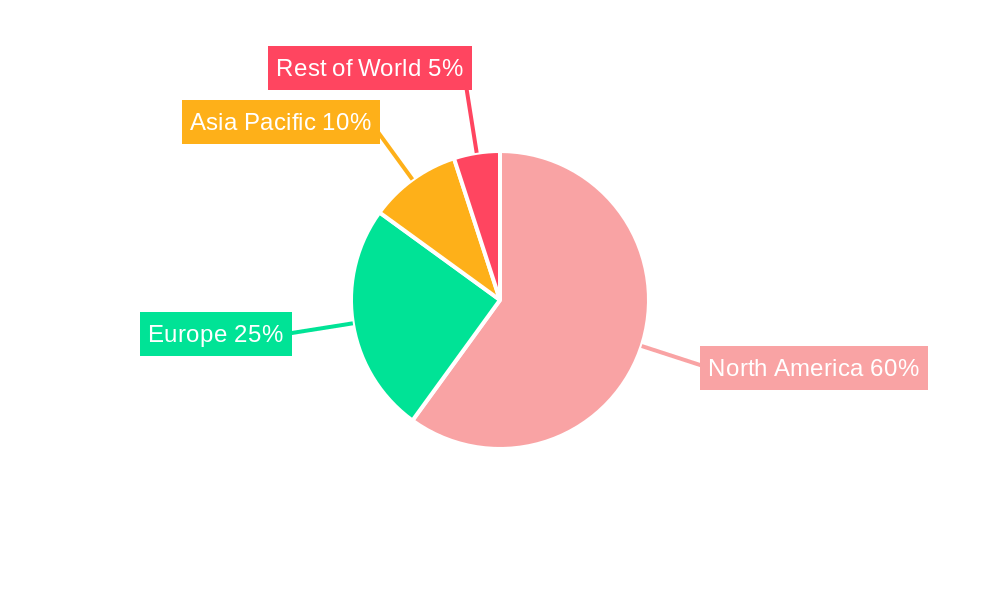
North America Autoimmune Disease Diagnostics Market Regional Market Share

Geographic Coverage of North America Autoimmune Disease Diagnostics Market
North America Autoimmune Disease Diagnostics Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 5.53% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Prevalence and Growing Public Awareness of Autoimmune Diseases; Technological Advancements in Autoimmune Disease Diagnostics
- 3.3. Market Restrains
- 3.3.1. Slow Turnaround Time for Autoimmune Disease Diagnostic Test Results; High Frequency of False Positive Result
- 3.4. Market Trends
- 3.4.1. Immunologic Assays Segment is Expected to Hold a Significant Market Share Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. North America Autoimmune Disease Diagnostics Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Disease Type
- 5.1.1. Systemic Autoimmune Disease
- 5.1.1.1. Rheumatoid Arthritis
- 5.1.1.2. Psoriasis
- 5.1.1.3. Systemic Lupus Erythematosus (SLE)
- 5.1.1.4. Multiple Sclerosis
- 5.1.1.5. Other Disease Types
- 5.1.2. Localized Autoimmune Disease
- 5.1.2.1. Inflammatory Bowel disease
- 5.1.2.2. Type 1 Diabetes
- 5.1.2.3. Thyroid
- 5.1.2.4. Other Localized Autoimmune Diseases
- 5.1.1. Systemic Autoimmune Disease
- 5.2. Market Analysis, Insights and Forecast - by Diagnostic Test
- 5.2.1. Regular Laboratory Tests
- 5.2.2. Inflammatory Markers
- 5.2.3. Immunologic Assays
- 5.2.4. Antibody Tests
- 5.2.5. Other Tests
- 5.3. Market Analysis, Insights and Forecast - by Geography
- 5.3.1. United States
- 5.3.2. Canada
- 5.3.3. Mexico
- 5.4. Market Analysis, Insights and Forecast - by Region
- 5.4.1. United States
- 5.4.2. Canada
- 5.4.3. Mexico
- 5.1. Market Analysis, Insights and Forecast - by Disease Type
- 6. United States North America Autoimmune Disease Diagnostics Market Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Disease Type
- 6.1.1. Systemic Autoimmune Disease
- 6.1.1.1. Rheumatoid Arthritis
- 6.1.1.2. Psoriasis
- 6.1.1.3. Systemic Lupus Erythematosus (SLE)
- 6.1.1.4. Multiple Sclerosis
- 6.1.1.5. Other Disease Types
- 6.1.2. Localized Autoimmune Disease
- 6.1.2.1. Inflammatory Bowel disease
- 6.1.2.2. Type 1 Diabetes
- 6.1.2.3. Thyroid
- 6.1.2.4. Other Localized Autoimmune Diseases
- 6.1.1. Systemic Autoimmune Disease
- 6.2. Market Analysis, Insights and Forecast - by Diagnostic Test
- 6.2.1. Regular Laboratory Tests
- 6.2.2. Inflammatory Markers
- 6.2.3. Immunologic Assays
- 6.2.4. Antibody Tests
- 6.2.5. Other Tests
- 6.3. Market Analysis, Insights and Forecast - by Geography
- 6.3.1. United States
- 6.3.2. Canada
- 6.3.3. Mexico
- 6.1. Market Analysis, Insights and Forecast - by Disease Type
- 7. Canada North America Autoimmune Disease Diagnostics Market Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Disease Type
- 7.1.1. Systemic Autoimmune Disease
- 7.1.1.1. Rheumatoid Arthritis
- 7.1.1.2. Psoriasis
- 7.1.1.3. Systemic Lupus Erythematosus (SLE)
- 7.1.1.4. Multiple Sclerosis
- 7.1.1.5. Other Disease Types
- 7.1.2. Localized Autoimmune Disease
- 7.1.2.1. Inflammatory Bowel disease
- 7.1.2.2. Type 1 Diabetes
- 7.1.2.3. Thyroid
- 7.1.2.4. Other Localized Autoimmune Diseases
- 7.1.1. Systemic Autoimmune Disease
- 7.2. Market Analysis, Insights and Forecast - by Diagnostic Test
- 7.2.1. Regular Laboratory Tests
- 7.2.2. Inflammatory Markers
- 7.2.3. Immunologic Assays
- 7.2.4. Antibody Tests
- 7.2.5. Other Tests
- 7.3. Market Analysis, Insights and Forecast - by Geography
- 7.3.1. United States
- 7.3.2. Canada
- 7.3.3. Mexico
- 7.1. Market Analysis, Insights and Forecast - by Disease Type
- 8. Mexico North America Autoimmune Disease Diagnostics Market Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Disease Type
- 8.1.1. Systemic Autoimmune Disease
- 8.1.1.1. Rheumatoid Arthritis
- 8.1.1.2. Psoriasis
- 8.1.1.3. Systemic Lupus Erythematosus (SLE)
- 8.1.1.4. Multiple Sclerosis
- 8.1.1.5. Other Disease Types
- 8.1.2. Localized Autoimmune Disease
- 8.1.2.1. Inflammatory Bowel disease
- 8.1.2.2. Type 1 Diabetes
- 8.1.2.3. Thyroid
- 8.1.2.4. Other Localized Autoimmune Diseases
- 8.1.1. Systemic Autoimmune Disease
- 8.2. Market Analysis, Insights and Forecast - by Diagnostic Test
- 8.2.1. Regular Laboratory Tests
- 8.2.2. Inflammatory Markers
- 8.2.3. Immunologic Assays
- 8.2.4. Antibody Tests
- 8.2.5. Other Tests
- 8.3. Market Analysis, Insights and Forecast - by Geography
- 8.3.1. United States
- 8.3.2. Canada
- 8.3.3. Mexico
- 8.1. Market Analysis, Insights and Forecast - by Disease Type
- 9. Competitive Analysis
- 9.1. Market Share Analysis 2025
- 9.2. Company Profiles
- 9.2.1 Oncimmune
- 9.2.1.1. Overview
- 9.2.1.2. Products
- 9.2.1.3. SWOT Analysis
- 9.2.1.4. Recent Developments
- 9.2.1.5. Financials (Based on Availability)
- 9.2.2 HYCOR Biomedical
- 9.2.2.1. Overview
- 9.2.2.2. Products
- 9.2.2.3. SWOT Analysis
- 9.2.2.4. Recent Developments
- 9.2.2.5. Financials (Based on Availability)
- 9.2.3 Grifols SA
- 9.2.3.1. Overview
- 9.2.3.2. Products
- 9.2.3.3. SWOT Analysis
- 9.2.3.4. Recent Developments
- 9.2.3.5. Financials (Based on Availability)
- 9.2.4 Werfen Group
- 9.2.4.1. Overview
- 9.2.4.2. Products
- 9.2.4.3. SWOT Analysis
- 9.2.4.4. Recent Developments
- 9.2.4.5. Financials (Based on Availability)
- 9.2.5 Abbott Laboratories
- 9.2.5.1. Overview
- 9.2.5.2. Products
- 9.2.5.3. SWOT Analysis
- 9.2.5.4. Recent Developments
- 9.2.5.5. Financials (Based on Availability)
- 9.2.6 Siemens Healthineers Inc
- 9.2.6.1. Overview
- 9.2.6.2. Products
- 9.2.6.3. SWOT Analysis
- 9.2.6.4. Recent Developments
- 9.2.6.5. Financials (Based on Availability)
- 9.2.7 Bio-rad Laboratories
- 9.2.7.1. Overview
- 9.2.7.2. Products
- 9.2.7.3. SWOT Analysis
- 9.2.7.4. Recent Developments
- 9.2.7.5. Financials (Based on Availability)
- 9.2.8 Myriad Genetics
- 9.2.8.1. Overview
- 9.2.8.2. Products
- 9.2.8.3. SWOT Analysis
- 9.2.8.4. Recent Developments
- 9.2.8.5. Financials (Based on Availability)
- 9.2.9 Euroimmun AG (Perkinelmer Inc )
- 9.2.9.1. Overview
- 9.2.9.2. Products
- 9.2.9.3. SWOT Analysis
- 9.2.9.4. Recent Developments
- 9.2.9.5. Financials (Based on Availability)
- 9.2.10 F Hoffmann-la Roche
- 9.2.10.1. Overview
- 9.2.10.2. Products
- 9.2.10.3. SWOT Analysis
- 9.2.10.4. Recent Developments
- 9.2.10.5. Financials (Based on Availability)
- 9.2.11 Thermo Fisher Scientific
- 9.2.11.1. Overview
- 9.2.11.2. Products
- 9.2.11.3. SWOT Analysis
- 9.2.11.4. Recent Developments
- 9.2.11.5. Financials (Based on Availability)
- 9.2.12 Trinity Biotech
- 9.2.12.1. Overview
- 9.2.12.2. Products
- 9.2.12.3. SWOT Analysis
- 9.2.12.4. Recent Developments
- 9.2.12.5. Financials (Based on Availability)
- 9.2.1 Oncimmune
List of Figures
- Figure 1: North America Autoimmune Disease Diagnostics Market Revenue Breakdown (Million, %) by Product 2025 & 2033
- Figure 2: North America Autoimmune Disease Diagnostics Market Share (%) by Company 2025
List of Tables
- Table 1: North America Autoimmune Disease Diagnostics Market Revenue Million Forecast, by Disease Type 2020 & 2033
- Table 2: North America Autoimmune Disease Diagnostics Market Volume K Unit Forecast, by Disease Type 2020 & 2033
- Table 3: North America Autoimmune Disease Diagnostics Market Revenue Million Forecast, by Diagnostic Test 2020 & 2033
- Table 4: North America Autoimmune Disease Diagnostics Market Volume K Unit Forecast, by Diagnostic Test 2020 & 2033
- Table 5: North America Autoimmune Disease Diagnostics Market Revenue Million Forecast, by Geography 2020 & 2033
- Table 6: North America Autoimmune Disease Diagnostics Market Volume K Unit Forecast, by Geography 2020 & 2033
- Table 7: North America Autoimmune Disease Diagnostics Market Revenue Million Forecast, by Region 2020 & 2033
- Table 8: North America Autoimmune Disease Diagnostics Market Volume K Unit Forecast, by Region 2020 & 2033
- Table 9: North America Autoimmune Disease Diagnostics Market Revenue Million Forecast, by Disease Type 2020 & 2033
- Table 10: North America Autoimmune Disease Diagnostics Market Volume K Unit Forecast, by Disease Type 2020 & 2033
- Table 11: North America Autoimmune Disease Diagnostics Market Revenue Million Forecast, by Diagnostic Test 2020 & 2033
- Table 12: North America Autoimmune Disease Diagnostics Market Volume K Unit Forecast, by Diagnostic Test 2020 & 2033
- Table 13: North America Autoimmune Disease Diagnostics Market Revenue Million Forecast, by Geography 2020 & 2033
- Table 14: North America Autoimmune Disease Diagnostics Market Volume K Unit Forecast, by Geography 2020 & 2033
- Table 15: North America Autoimmune Disease Diagnostics Market Revenue Million Forecast, by Country 2020 & 2033
- Table 16: North America Autoimmune Disease Diagnostics Market Volume K Unit Forecast, by Country 2020 & 2033
- Table 17: North America Autoimmune Disease Diagnostics Market Revenue Million Forecast, by Disease Type 2020 & 2033
- Table 18: North America Autoimmune Disease Diagnostics Market Volume K Unit Forecast, by Disease Type 2020 & 2033
- Table 19: North America Autoimmune Disease Diagnostics Market Revenue Million Forecast, by Diagnostic Test 2020 & 2033
- Table 20: North America Autoimmune Disease Diagnostics Market Volume K Unit Forecast, by Diagnostic Test 2020 & 2033
- Table 21: North America Autoimmune Disease Diagnostics Market Revenue Million Forecast, by Geography 2020 & 2033
- Table 22: North America Autoimmune Disease Diagnostics Market Volume K Unit Forecast, by Geography 2020 & 2033
- Table 23: North America Autoimmune Disease Diagnostics Market Revenue Million Forecast, by Country 2020 & 2033
- Table 24: North America Autoimmune Disease Diagnostics Market Volume K Unit Forecast, by Country 2020 & 2033
- Table 25: North America Autoimmune Disease Diagnostics Market Revenue Million Forecast, by Disease Type 2020 & 2033
- Table 26: North America Autoimmune Disease Diagnostics Market Volume K Unit Forecast, by Disease Type 2020 & 2033
- Table 27: North America Autoimmune Disease Diagnostics Market Revenue Million Forecast, by Diagnostic Test 2020 & 2033
- Table 28: North America Autoimmune Disease Diagnostics Market Volume K Unit Forecast, by Diagnostic Test 2020 & 2033
- Table 29: North America Autoimmune Disease Diagnostics Market Revenue Million Forecast, by Geography 2020 & 2033
- Table 30: North America Autoimmune Disease Diagnostics Market Volume K Unit Forecast, by Geography 2020 & 2033
- Table 31: North America Autoimmune Disease Diagnostics Market Revenue Million Forecast, by Country 2020 & 2033
- Table 32: North America Autoimmune Disease Diagnostics Market Volume K Unit Forecast, by Country 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the North America Autoimmune Disease Diagnostics Market?
The projected CAGR is approximately 5.53%.
2. Which companies are prominent players in the North America Autoimmune Disease Diagnostics Market?
Key companies in the market include Oncimmune, HYCOR Biomedical, Grifols SA, Werfen Group, Abbott Laboratories, Siemens Healthineers Inc, Bio-rad Laboratories, Myriad Genetics, Euroimmun AG (Perkinelmer Inc ), F Hoffmann-la Roche, Thermo Fisher Scientific, Trinity Biotech.
3. What are the main segments of the North America Autoimmune Disease Diagnostics Market?
The market segments include Disease Type, Diagnostic Test, Geography.
4. Can you provide details about the market size?
The market size is estimated to be USD 2.05 Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Prevalence and Growing Public Awareness of Autoimmune Diseases; Technological Advancements in Autoimmune Disease Diagnostics.
6. What are the notable trends driving market growth?
Immunologic Assays Segment is Expected to Hold a Significant Market Share Over the Forecast Period.
7. Are there any restraints impacting market growth?
Slow Turnaround Time for Autoimmune Disease Diagnostic Test Results; High Frequency of False Positive Result.
8. Can you provide examples of recent developments in the market?
February 2023: Edesa Biotech received approval from Health Canada for a phase II clinical trial of its EB06 monoclonal antibody candidate to treat vitiligo, a life-altering autoimmune disease.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "North America Autoimmune Disease Diagnostics Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the North America Autoimmune Disease Diagnostics Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the North America Autoimmune Disease Diagnostics Market?
To stay informed about further developments, trends, and reports in the North America Autoimmune Disease Diagnostics Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
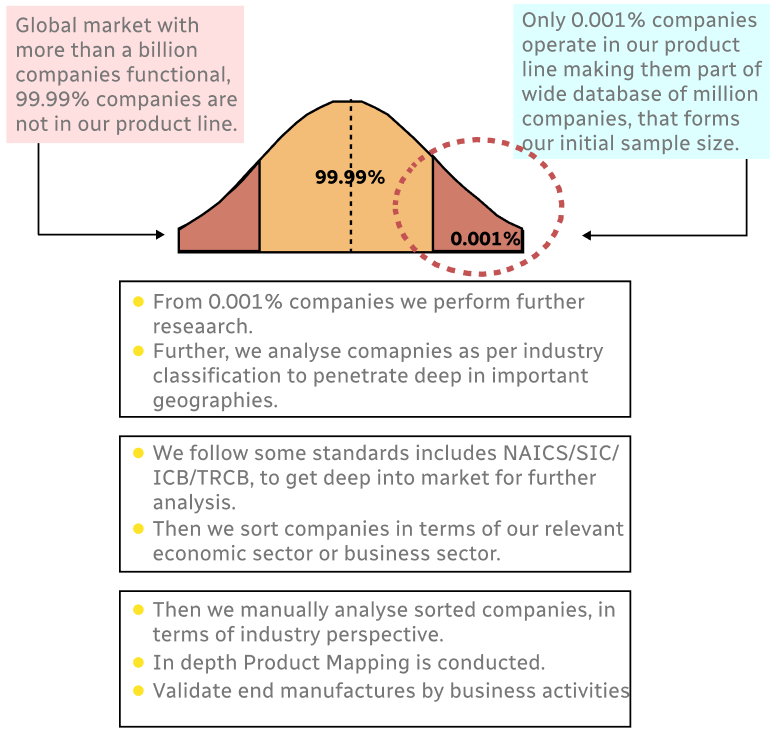
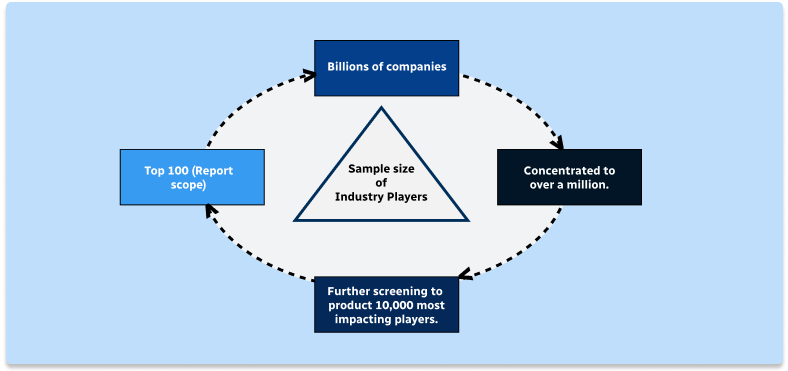
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
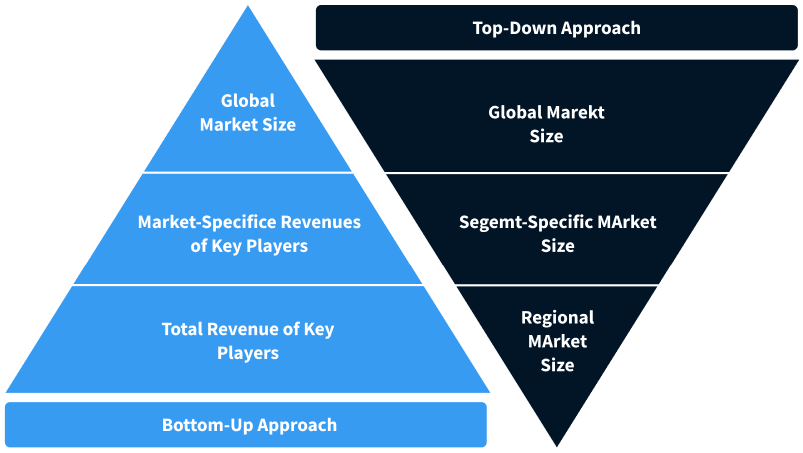
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
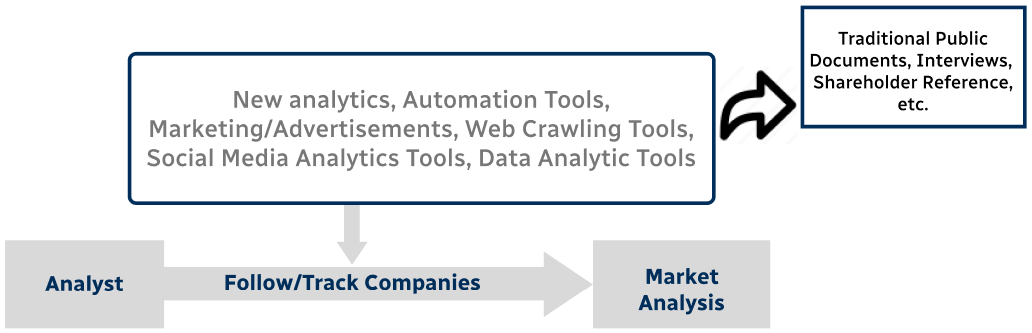
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


