Key Insights
The Asia-Pacific Contract Development and Manufacturing Organization (CDMO) market is experiencing robust growth, projected to reach a market size of $71.60 billion in 2025, expanding at a Compound Annual Growth Rate (CAGR) of 8.60% from 2025 to 2033. This significant expansion is fueled by several key factors. The burgeoning pharmaceutical and biotechnology industries in the region, particularly in China, India, and Japan, are driving increased demand for CDMO services. The rising prevalence of chronic diseases and a growing aging population necessitate the development and manufacturing of innovative therapeutics, further stimulating the market. Furthermore, the increasing adoption of outsourcing strategies by pharmaceutical companies to reduce operational costs and focus on core competencies contributes to the market's growth. The robust growth of the biosimilars market adds another layer to the expanding demand for CDMO services, encompassing high-potency APIs (HPAPIs), finished dosage formulations (FDFs), and diverse dosage forms such as tablets and injectables.
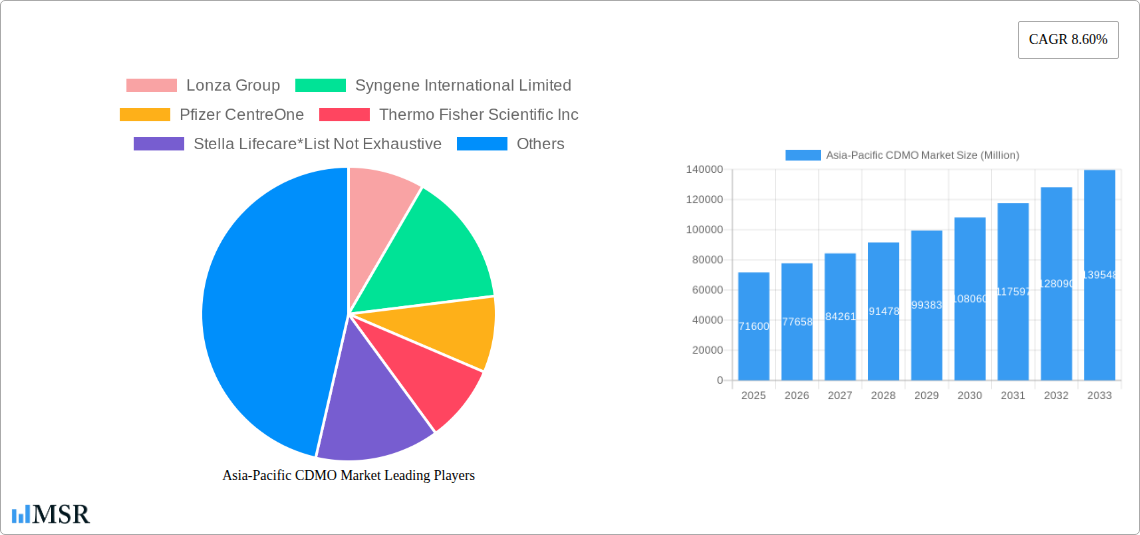
Asia-Pacific CDMO Market Market Size (In Billion)

Market segmentation reveals the dominance of specific service types and countries. Active Pharmaceutical Ingredient (API) manufacturing, particularly HPAPI, and finished dosage formulation development and manufacturing are major revenue generators. Within geographical segments, China, Japan, and India represent the largest markets within the Asia-Pacific region, owing to their established manufacturing infrastructure and growing domestic pharmaceutical sectors. While the exact market shares for each country are not explicitly provided, it can be inferred that China, due to its size and manufacturing capabilities, holds a substantial share, followed by India and Japan. The market's growth will likely continue to be influenced by regulatory changes, technological advancements in drug delivery systems, and increasing investments in research and development within the Asia-Pacific region. The presence of numerous established and emerging CDMO players further contributes to the dynamic and competitive landscape.
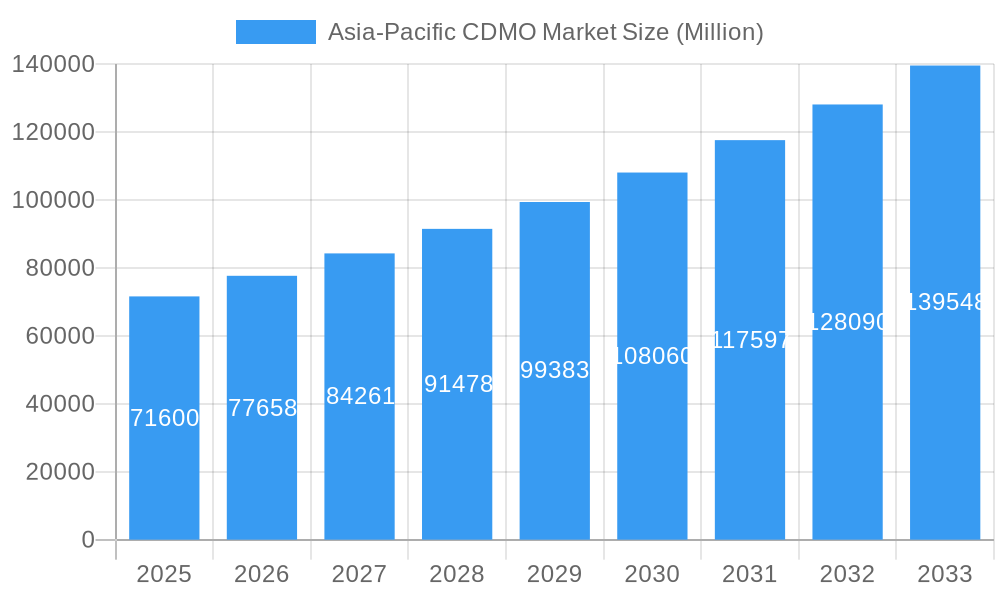
Asia-Pacific CDMO Market Company Market Share

Asia-Pacific CDMO Market Report: 2019-2033
This comprehensive report provides an in-depth analysis of the Asia-Pacific Contract Development and Manufacturing Organization (CDMO) market, covering the period from 2019 to 2033. It offers actionable insights for stakeholders, including pharmaceutical companies, investors, and CDMO providers, seeking to navigate this rapidly evolving landscape. The report forecasts significant growth, driven by technological advancements, increasing outsourcing, and a burgeoning pharmaceutical industry across the region. Key players like Lonza Group, WuXi Biologics, and Samsung Biologics are shaping this dynamic market. This report analyzes market trends, key segments, competitive landscape, and future opportunities, providing a crucial roadmap for success in the Asia-Pacific CDMO sector.
Asia-Pacific CDMO Market Market Concentration & Dynamics
The Asia-Pacific CDMO market exhibits a moderately concentrated landscape, with a few large players holding significant market share. However, the presence of numerous smaller, specialized CDMOs fosters competition and innovation. Market share data for 2025 estimates WuXi Biologics at approximately xx%, followed by Samsung Biologics at xx%, and Lonza Group at xx%. The remaining share is distributed among other players including Catalent Inc, FUJIFILM Diosynth Biotechnologies and others. Innovation ecosystems are concentrated in key hubs like China, Japan, and India, supported by robust R&D investments and government initiatives. Regulatory frameworks vary across countries, impacting market access and operational complexities. Substitute products include in-house manufacturing, but the trend favors outsourcing due to cost efficiency and access to specialized expertise. End-user trends reveal a shift towards integrated CDMOs offering a broader range of services, from drug discovery to commercial manufacturing. M&A activity has been significant, with xx deals recorded between 2019 and 2024, reflecting consolidation and expansion strategies. The average deal size was approximately $xx Million.
Asia-Pacific CDMO Market Industry Insights & Trends
The Asia-Pacific CDMO market is experiencing robust growth, with a Compound Annual Growth Rate (CAGR) of xx% projected from 2025 to 2033. The market size in 2025 is estimated at $xx Million, and is expected to reach $xx Million by 2033. This expansion is primarily driven by factors such as the increasing prevalence of chronic diseases, rising demand for biopharmaceuticals, and the growing adoption of outsourcing by pharmaceutical companies. Technological advancements, particularly in areas like cell and gene therapy and advanced analytics, are further fueling market growth. Evolving consumer behaviors, such as increased health awareness and demand for innovative therapies, contribute to the growing demand for CDMO services. The rising investment in R&D by both government and private players is a significant positive impact. Regulatory changes aiming to streamline drug approval processes are also supporting market expansion.
Key Markets & Segments Leading Asia-Pacific CDMO Market
-
Dominant Regions/Countries: China continues to command the largest market share in the Asia-Pacific CDMO market, significantly influenced by its rapidly expanding pharmaceutical sector, substantial government backing, and a cost-effective manufacturing infrastructure. Following closely are Japan and India. Japan distinguishes itself with its stringent quality manufacturing standards, advanced technological capabilities, and a robust commitment to pharmaceutical innovation. India's prominence is fueled by its thriving generic drug manufacturing industry, considerable cost advantages, and an extensive pool of highly skilled scientists and engineers. While Australia and New Zealand currently represent smaller market segments, they are demonstrating promising growth, attracting increased investments and developing sophisticated capabilities within the sector.
-
Dominant Service Type CDMO Segments:
- Active Pharmaceutical Ingredient (API) Manufacturing: This segment remains a cornerstone of the CDMO market, experiencing sustained high demand driven by the strategic outsourcing of API production by global pharmaceutical companies seeking specialized expertise and economies of scale.
- High Potency (HPAPI) Finished Dosage Formulation (FDF) Development and Manufacturing: This specialized and rapidly expanding niche is propelled by the escalating demand for HPAPIs, particularly in the development of advanced oncology treatments and other complex therapeutic areas.
- Solid Dose Formulation (Tablets and Capsules): This remains a large, stable, and consistently in-demand segment, forming a significant portion of the CDMO service offering for a wide range of pharmaceutical products.
- Injectable Dose Formulation: This segment is witnessing substantial growth, underscored by the increasing prevalence and development of injectable drug therapies, requiring specialized aseptic manufacturing capabilities.
- Biologics Manufacturing: A rapidly growing area, driven by the surge in demand for complex biological drugs, including monoclonal antibodies, vaccines, and recombinant proteins. CDMOs with expertise in bioprocessing and large-scale fermentation are well-positioned.
- Cell and Gene Therapy Services: This cutting-edge segment is experiencing exponential growth. CDMOs offering specialized manufacturing and development services for cell and gene therapies are in high demand, reflecting the transformative potential of these novel treatment modalities.
- Secondary Packaging: While often seen as a supporting service, secondary packaging represents a critical aspect of the market. Its continued growth is projected as it ensures product integrity, regulatory compliance, and market readiness for a diverse range of pharmaceutical products.
-
Dominant Research and Development (R&D) Phase Segments: All phases of pharmaceutical R&D, from early-stage preclinical studies through to post-market Phase IV evaluations, are integral components of the CDMO service landscape. However, there is a pronounced demand in the early phases, encompassing preclinical research and Phase I clinical trials. This surge is directly attributable to the continuous influx of novel drug candidates entering the development pipeline, requiring specialized expertise and infrastructure for their initial assessment and validation.
Asia-Pacific CDMO Market Product Developments
The Asia-Pacific CDMO market is characterized by continuous product innovation, focusing on advanced technologies and specialized services. Recent developments include the adoption of continuous manufacturing processes for improved efficiency and reduced costs, the expansion of cell and gene therapy manufacturing capabilities, and the increasing integration of advanced analytical techniques for process optimization and quality control. These innovations are enhancing the speed, efficiency, and quality of drug development and manufacturing, creating a significant competitive edge for CDMOs.
Challenges in the Asia-Pacific CDMO Market Market
The Asia-Pacific CDMO market faces several challenges, including regulatory complexities that vary across countries, increasing pressure on pricing and margins due to competition, and ensuring consistent quality and supply chain management across the diverse geographical region. These challenges, if not addressed effectively, could impact the market growth trajectory. Supply chain disruptions arising from global events can lead to delays and shortages of essential materials impacting productivity and timelines. Furthermore, meeting the rigorous regulatory standards across different countries can prove complex and expensive.
Forces Driving Asia-Pacific CDMO Market Growth
Multiple potent forces are propelling the expansion of the Asia-Pacific CDMO market. A primary driver is the escalating trend of outsourcing drug development and manufacturing by pharmaceutical and biotechnology companies, both large and small, seeking specialized expertise, cost efficiencies, and enhanced agility. Significant investments in research and development (R&D) across the region, coupled with the robust growth of the biopharmaceutical industry, are creating substantial demand for CDMO services. Favorable government regulations and incentives in many Asia-Pacific nations further foster an environment conducive to growth. Technological advancements, particularly in innovative areas like continuous manufacturing, advanced process development, and the burgeoning field of cell and gene therapy manufacturing, are also key contributors. The overarching economic growth observed in many Asia-Pacific countries is directly translating into increased investment and expansion within the CDMO sector.
Challenges in the Asia-Pacific CDMO Market Market
Long-term growth catalysts include the continuous evolution of technology, strategic partnerships between CDMOs and pharmaceutical companies, and expansion into new therapeutic areas such as cell and gene therapy, and continuous efforts to meet increased demand and maintain high quality standards. Further expansion into emerging markets within the region are driving forces to consider.
Emerging Opportunities in Asia-Pacific CDMO Market
Emerging opportunities include the growth of the biosimilars market, increasing demand for personalized medicine, and the expansion into new therapeutic areas such as cell and gene therapy. The development of advanced analytical technologies and digitalization are also creating opportunities for increased efficiency and improved decision-making within the CDMO sector. Focusing on niche therapeutic areas and establishing strategic partnerships are key strategies to capture opportunities in this sector.
Leading Players in the Asia-Pacific CDMO Market Sector
- Lonza Group
- Syngene International Limited
- Pfizer CentreOne
- Thermo Fisher Scientific Inc
- Stella Lifecare
- WuXi Biologics
- Catalent Inc
- FUJIFILM Diosynth Biotechnologies
- Samsung Biologics
- Jubilant Biosys Ltd
- Boehringer Ingelheim Group
- Recipharm AB
Key Milestones in Asia-Pacific CDMO Market Industry
- September 2023: WuXi Vaccines launches a new standalone vaccines CDMO site in Suzhou, China, expanding capacity for drug substances and products.
- March 2023: Samsung Biologics announces the development of a fifth facility, representing a significant investment in expanding capacity to meet market demand.
Strategic Outlook for Asia-Pacific CDMO Market Market
The Asia-Pacific CDMO market is poised for continued strong growth, driven by technological innovation, strategic partnerships, and expanding demand from the biopharmaceutical industry. Companies focusing on advanced technologies, specialized services, and efficient supply chain management will be best positioned to capitalize on the market's future potential. Strategic investments in capacity expansion, R&D, and talent acquisition will be crucial for maintaining a competitive edge in this dynamic market.
Asia-Pacific CDMO Market Segmentation
-
1. Service Type CMO Segment
-
1.1. Active P
- 1.1.1. Small Molecule
- 1.1.2. Large Molecule
- 1.1.3. High Potency (HPAPI)
-
1.2. Finished
-
1.2.1. Solid Dose Formulation
- 1.2.1.1. Tablets
- 1.2.1.2. Others
- 1.2.2. Liquid Dose Formulation
- 1.2.3. Injectable Dose Formulation
-
1.2.1. Solid Dose Formulation
- 1.3. Secondary Packaging
-
1.1. Active P
-
2. Research Phase CRO Segment
- 2.1. Pre-clinical
- 2.2. Phase I
- 2.3. Phase II
- 2.4. Phase III
- 2.5. Phase IV
Asia-Pacific CDMO Market Segmentation By Geography
-
1. Asia Pacific
- 1.1. China
- 1.2. Japan
- 1.3. South Korea
- 1.4. India
- 1.5. Australia
- 1.6. New Zealand
- 1.7. Indonesia
- 1.8. Malaysia
- 1.9. Singapore
- 1.10. Thailand
- 1.11. Vietnam
- 1.12. Philippines
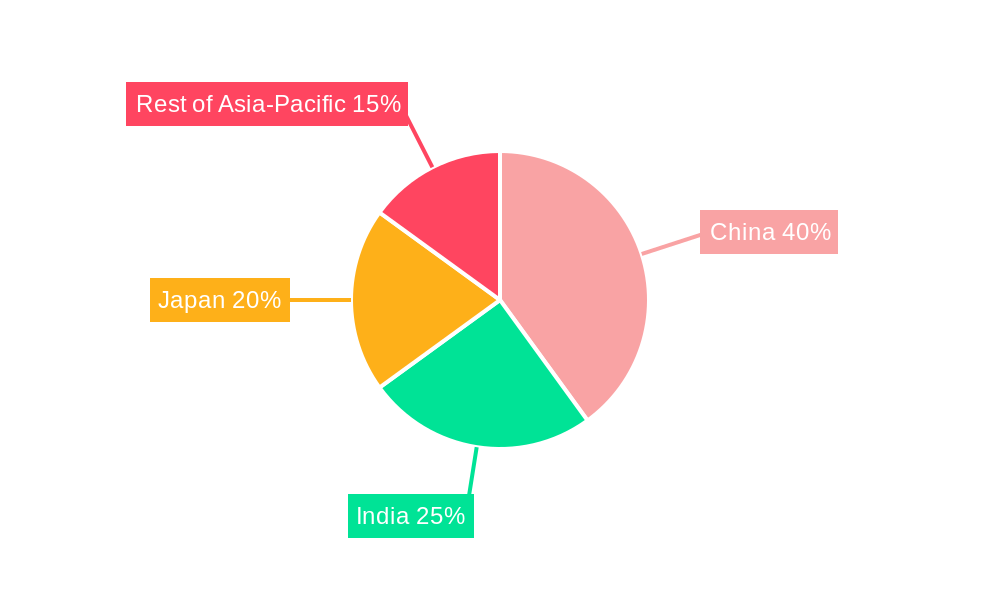
Asia-Pacific CDMO Market Regional Market Share

Geographic Coverage of Asia-Pacific CDMO Market
Asia-Pacific CDMO Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 8.60% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. 4.; Increasing Outsourcing Volume by Big Pharmaceutical Companies4.; Increasing Investment in Research and Development
- 3.3. Market Restrains
- 3.3.1. 4.; Increasing Lead Time Owing to Supply Chain Related Constraints in the Region4.; Skilled Labour Shortages Across the Region
- 3.4. Market Trends
- 3.4.1. The Demand For Injectable Dose Formulation is Rising in the Market
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Asia-Pacific CDMO Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 5.1.1. Active P
- 5.1.1.1. Small Molecule
- 5.1.1.2. Large Molecule
- 5.1.1.3. High Potency (HPAPI)
- 5.1.2. Finished
- 5.1.2.1. Solid Dose Formulation
- 5.1.2.1.1. Tablets
- 5.1.2.1.2. Others
- 5.1.2.2. Liquid Dose Formulation
- 5.1.2.3. Injectable Dose Formulation
- 5.1.2.1. Solid Dose Formulation
- 5.1.3. Secondary Packaging
- 5.1.1. Active P
- 5.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 5.2.1. Pre-clinical
- 5.2.2. Phase I
- 5.2.3. Phase II
- 5.2.4. Phase III
- 5.2.5. Phase IV
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 6. Competitive Analysis
- 6.1. Market Share Analysis 2025
- 6.2. Company Profiles
- 6.2.1 Lonza Group
- 6.2.1.1. Overview
- 6.2.1.2. Products
- 6.2.1.3. SWOT Analysis
- 6.2.1.4. Recent Developments
- 6.2.1.5. Financials (Based on Availability)
- 6.2.2 Syngene International Limited
- 6.2.2.1. Overview
- 6.2.2.2. Products
- 6.2.2.3. SWOT Analysis
- 6.2.2.4. Recent Developments
- 6.2.2.5. Financials (Based on Availability)
- 6.2.3 Pfizer CentreOne
- 6.2.3.1. Overview
- 6.2.3.2. Products
- 6.2.3.3. SWOT Analysis
- 6.2.3.4. Recent Developments
- 6.2.3.5. Financials (Based on Availability)
- 6.2.4 Thermo Fisher Scientific Inc
- 6.2.4.1. Overview
- 6.2.4.2. Products
- 6.2.4.3. SWOT Analysis
- 6.2.4.4. Recent Developments
- 6.2.4.5. Financials (Based on Availability)
- 6.2.5 Stella Lifecare*List Not Exhaustive
- 6.2.5.1. Overview
- 6.2.5.2. Products
- 6.2.5.3. SWOT Analysis
- 6.2.5.4. Recent Developments
- 6.2.5.5. Financials (Based on Availability)
- 6.2.6 WuXi Biologics
- 6.2.6.1. Overview
- 6.2.6.2. Products
- 6.2.6.3. SWOT Analysis
- 6.2.6.4. Recent Developments
- 6.2.6.5. Financials (Based on Availability)
- 6.2.7 Catalent Inc
- 6.2.7.1. Overview
- 6.2.7.2. Products
- 6.2.7.3. SWOT Analysis
- 6.2.7.4. Recent Developments
- 6.2.7.5. Financials (Based on Availability)
- 6.2.8 FUJIFILM Diosynth Biotechnologies
- 6.2.8.1. Overview
- 6.2.8.2. Products
- 6.2.8.3. SWOT Analysis
- 6.2.8.4. Recent Developments
- 6.2.8.5. Financials (Based on Availability)
- 6.2.9 Samsung Biologics
- 6.2.9.1. Overview
- 6.2.9.2. Products
- 6.2.9.3. SWOT Analysis
- 6.2.9.4. Recent Developments
- 6.2.9.5. Financials (Based on Availability)
- 6.2.10 Jubilant Biosys Ltd
- 6.2.10.1. Overview
- 6.2.10.2. Products
- 6.2.10.3. SWOT Analysis
- 6.2.10.4. Recent Developments
- 6.2.10.5. Financials (Based on Availability)
- 6.2.11 Boehringer Ingelheim Group
- 6.2.11.1. Overview
- 6.2.11.2. Products
- 6.2.11.3. SWOT Analysis
- 6.2.11.4. Recent Developments
- 6.2.11.5. Financials (Based on Availability)
- 6.2.12 Recipharm AB
- 6.2.12.1. Overview
- 6.2.12.2. Products
- 6.2.12.3. SWOT Analysis
- 6.2.12.4. Recent Developments
- 6.2.12.5. Financials (Based on Availability)
- 6.2.1 Lonza Group
List of Figures
- Figure 1: Asia-Pacific CDMO Market Revenue Breakdown (Million, %) by Product 2025 & 2033
- Figure 2: Asia-Pacific CDMO Market Share (%) by Company 2025
List of Tables
- Table 1: Asia-Pacific CDMO Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 2: Asia-Pacific CDMO Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 3: Asia-Pacific CDMO Market Revenue Million Forecast, by Region 2020 & 2033
- Table 4: Asia-Pacific CDMO Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 5: Asia-Pacific CDMO Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 6: Asia-Pacific CDMO Market Revenue Million Forecast, by Country 2020 & 2033
- Table 7: China Asia-Pacific CDMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 8: Japan Asia-Pacific CDMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 9: South Korea Asia-Pacific CDMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 10: India Asia-Pacific CDMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 11: Australia Asia-Pacific CDMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 12: New Zealand Asia-Pacific CDMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 13: Indonesia Asia-Pacific CDMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 14: Malaysia Asia-Pacific CDMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 15: Singapore Asia-Pacific CDMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 16: Thailand Asia-Pacific CDMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 17: Vietnam Asia-Pacific CDMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 18: Philippines Asia-Pacific CDMO Market Revenue (Million) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Asia-Pacific CDMO Market?
The projected CAGR is approximately 8.60%.
2. Which companies are prominent players in the Asia-Pacific CDMO Market?
Key companies in the market include Lonza Group, Syngene International Limited, Pfizer CentreOne, Thermo Fisher Scientific Inc, Stella Lifecare*List Not Exhaustive, WuXi Biologics, Catalent Inc, FUJIFILM Diosynth Biotechnologies, Samsung Biologics, Jubilant Biosys Ltd, Boehringer Ingelheim Group, Recipharm AB.
3. What are the main segments of the Asia-Pacific CDMO Market?
The market segments include Service Type CMO Segment, Research Phase CRO Segment.
4. Can you provide details about the market size?
The market size is estimated to be USD 71.60 Million as of 2022.
5. What are some drivers contributing to market growth?
4.; Increasing Outsourcing Volume by Big Pharmaceutical Companies4.; Increasing Investment in Research and Development.
6. What are the notable trends driving market growth?
The Demand For Injectable Dose Formulation is Rising in the Market.
7. Are there any restraints impacting market growth?
4.; Increasing Lead Time Owing to Supply Chain Related Constraints in the Region4.; Skilled Labour Shortages Across the Region.
8. Can you provide examples of recent developments in the market?
September 2023: WuXi Vaccines, a key pharmaceutical CDMO firm, introduced a standalone vaccines CDMO site in Suzhou, China. The expansion was expected to introduce enhanced capacity for both drug substances and drug products, offering comprehensive services for a range of vaccines. This move aimed to expedite project timelines for the company's global clients, covering everything from process and drug product development to manufacturing clinical-scale drug substances (DS) and small-to-medium sterile drug products (DP).
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 4950, and USD 6800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Asia-Pacific CDMO Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Asia-Pacific CDMO Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Asia-Pacific CDMO Market?
To stay informed about further developments, trends, and reports in the Asia-Pacific CDMO Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


