Key Insights
The North American pharmaceutical contract manufacturing organization (CMO) market is experiencing robust growth, driven by several key factors. The increasing complexity of drug development, coupled with the rising demand for specialized services like High Potency API (HPAPI) manufacturing and aseptic fill-finish capabilities, is fueling outsourcing to CMOs. Pharmaceutical companies are increasingly focusing on core competencies, leading to a significant shift towards strategic partnerships with CMOs to manage manufacturing and supply chain complexities. The market's expansion is further propelled by the growing pipeline of innovative therapies, particularly biologics and advanced therapies, which often require specialized manufacturing expertise beyond the capabilities of many pharmaceutical companies. This trend is particularly pronounced in North America, which boasts a strong regulatory environment and a well-established network of experienced CMOs. The market is segmented by service type, encompassing API manufacturing, HPAPI and FDF development & manufacturing, and injectable dose formulation and secondary packaging. Geographic segmentation includes the United States and Canada, with the US holding the larger market share due to its significant pharmaceutical industry presence and higher R&D investment.
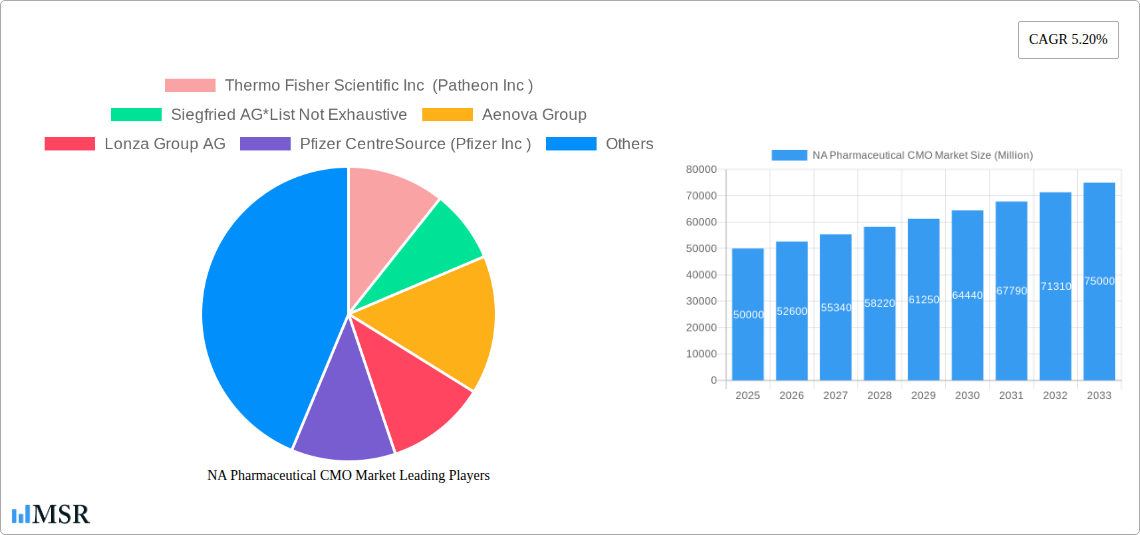
NA Pharmaceutical CMO Market Market Size (In Billion)

Considering the provided CAGR of 5.20% and a 2025 market size (estimated, as the exact figure is missing), let's assume a 2025 market size of $50 billion for the sake of illustration. This conservative estimate takes into account potential market fluctuations. Using this base, we can project future growth based on the CAGR. The projected growth signifies significant opportunities for established players like Thermo Fisher Scientific (Patheon), Siegfried AG, Lonza Group AG, and Catalent, as well as emerging CMOs striving to capture market share. Competition is intense, necessitating strategic investments in advanced technologies, capacity expansion, and regulatory compliance to maintain a competitive edge. The market will likely see consolidation as larger players acquire smaller companies to gain market share and expand service offerings. Furthermore, continuous innovation in manufacturing technologies and a focus on providing efficient and cost-effective solutions will be crucial for success in this dynamic market.
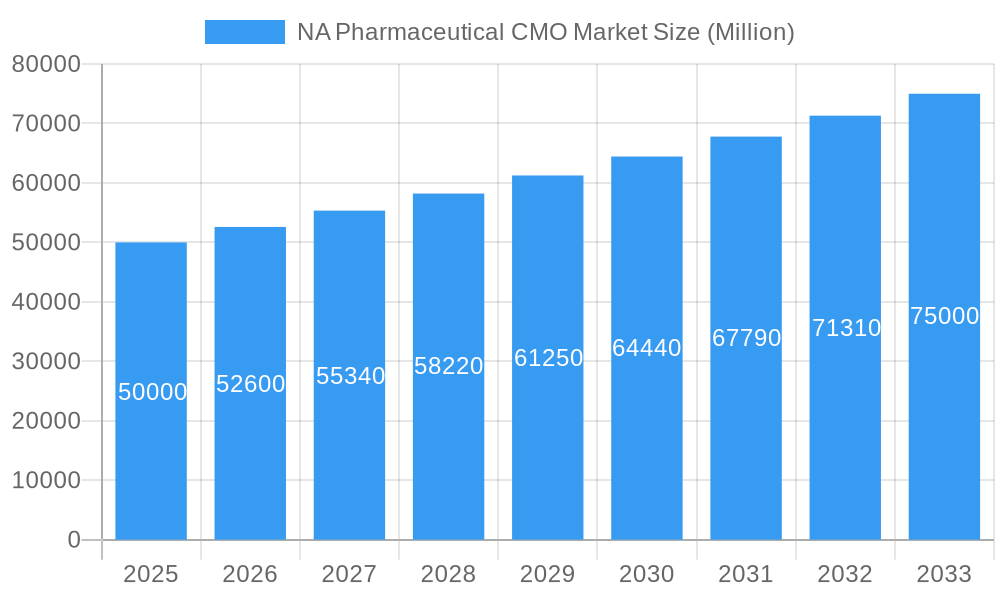
NA Pharmaceutical CMO Market Company Market Share

Unlock Growth in the North American Pharmaceutical CMO Market: A Comprehensive Report (2019-2033)
This in-depth report provides a comprehensive analysis of the North American (NA) Pharmaceutical Contract Manufacturing Organization (CMO) market, offering invaluable insights for stakeholders seeking to navigate this dynamic landscape. Covering the period from 2019 to 2033, with a base year of 2025 and a forecast period of 2025-2033, this report unveils key trends, challenges, and opportunities within the industry. The market is projected to reach xx Million by 2033, exhibiting a CAGR of xx% during the forecast period.
NA Pharmaceutical CMO Market Concentration & Dynamics
This section delves into the competitive intensity of the NA Pharmaceutical CMO market. We analyze market share distribution, identifying key players and their strategic positions. The report also examines the impact of mergers and acquisitions (M&A) activities, providing a detailed account of deal counts and their influence on market consolidation. Innovation ecosystems are assessed, highlighting the role of research and development in driving market growth. The regulatory landscape, including FDA guidelines and their implications, is thoroughly scrutinized. Furthermore, the report investigates substitute products and their potential to disrupt the market. Finally, evolving end-user trends and their impact on demand for CMO services are explored.
- Market Concentration: The NA Pharmaceutical CMO market exhibits a moderately concentrated structure, with the top 5 players holding an estimated xx% market share in 2025.
- M&A Activity: The historical period (2019-2024) witnessed xx M&A deals, indicating a significant level of consolidation. This trend is expected to continue during the forecast period, further shaping the market landscape.
- Innovation Ecosystems: Robust R&D activities and collaborations within the industry are driving innovation in API manufacturing, formulation development, and packaging technologies.
- Regulatory Framework: Stringent regulatory requirements imposed by the FDA significantly impact CMO operations and necessitate compliance with Good Manufacturing Practices (GMP).
- Substitute Products: The emergence of novel drug delivery systems and alternative manufacturing technologies presents potential substitutes to traditional CMO services.
- End-User Trends: Increasing demand for specialized drug products, including biologics and personalized medicines, is creating opportunities for CMOs with advanced capabilities.
NA Pharmaceutical CMO Market Industry Insights & Trends
This section provides a detailed analysis of the NA Pharmaceutical CMO market’s growth trajectory. We explore key drivers, including the rising prevalence of chronic diseases, the increasing outsourcing of pharmaceutical manufacturing, and the growing demand for cost-effective solutions. Technological advancements, such as automation and digitalization, are also analyzed for their impact on market efficiency and productivity. Furthermore, changing consumer behaviors, including increased awareness of drug safety and efficacy, are examined. The report also assesses market size and CAGR across various segments, providing a nuanced understanding of market dynamics. The total market size in 2025 is estimated to be xx Million, with a projected xx Million in 2033. The substantial market growth is driven by several factors including increasing investments in R&D, favorable government regulations, and technological advancements.
Key Markets & Segments Leading NA Pharmaceutical CMO Market
This section identifies the leading segments and regions within the NA Pharmaceutical CMO market. A detailed analysis is provided for the United States and Canada, considering key factors driving market dominance.
Dominant Segments:
- Active Pharmaceutical Ingredient (API) Manufacturing: This segment is anticipated to be the largest revenue contributor due to increasing demand for APIs from both large and small pharmaceutical companies.
- Finished Dosage Formulation (FDF) Development and Manufacturing: Growth is driven by the complexity of modern drug formulations and the need for specialized expertise in FDF development.
- Injectable Dose Formulation: The market for injectable drug formulations is witnessing steady growth, fueled by advancements in drug delivery technologies and the increasing prevalence of chronic diseases requiring injectable treatments.
Dominant Regions:
- United States: The US dominates the NA Pharmaceutical CMO market due to its large pharmaceutical industry, robust infrastructure, and presence of several major CMO players. Drivers include substantial R&D investments and the presence of key industry players.
- Canada: Canada shows potential growth due to its strong healthcare infrastructure and expanding pharmaceutical sector. However, its market size is significantly smaller compared to the United States.
NA Pharmaceutical CMO Market Product Developments
This report highlights recent product innovations, focusing on technological advancements that enhance manufacturing efficiency, improve product quality, and reduce costs. The discussion includes the impact of these developments on the competitive landscape. These innovations range from automation in manufacturing processes and advanced analytical techniques for quality control to the development of new drug delivery systems and personalized medicine approaches.
Challenges in the NA Pharmaceutical CMO Market Market
The NA Pharmaceutical CMO market faces several challenges, including stringent regulatory requirements leading to increased compliance costs and potential delays in product launch, supply chain disruptions caused by geopolitical events and pandemics, and intense competition among CMO providers, creating pricing pressures. These challenges, if not addressed effectively, could significantly impact market growth and profitability. For instance, supply chain issues in 2022 resulted in an estimated xx Million loss in revenue for the industry.
Forces Driving NA Pharmaceutical CMO Market Growth
The NA Pharmaceutical CMO market is poised for significant growth driven by several factors. Technological advancements leading to increased automation and efficiency in manufacturing processes, the continued outsourcing trend by pharmaceutical companies to reduce operational costs and gain access to specialized expertise, and favorable government regulations supporting the growth of the pharmaceutical industry are among the key drivers.
Long-Term Growth Catalysts in the NA Pharmaceutical CMO Market
Long-term growth in the NA Pharmaceutical CMO market is propelled by several factors. Continued investments in R&D and innovation, fostering advancements in drug delivery systems and manufacturing technologies, strategic partnerships and collaborations between CMOs and pharmaceutical companies to accelerate drug development and launch, and expansion into emerging markets, including personalized medicine and cell therapies, will fuel sustained growth.
Emerging Opportunities in NA Pharmaceutical CMO Market
Emerging opportunities in the NA Pharmaceutical CMO market include the increasing demand for complex drug products like biologics and advanced therapies, growth in the contract development and manufacturing (CDMO) services, expansion into emerging markets outside of North America, and the application of Artificial Intelligence (AI) and machine learning (ML) in optimizing manufacturing processes and quality control.
Leading Players in the NA Pharmaceutical CMO Market Sector
- Thermo Fisher Scientific Inc (Patheon Inc)
- Siegfried AG
- Aenova Group
- Lonza Group AG
- Pfizer CentreSource (Pfizer Inc)
- AbbVie Inc
- Jubilant Life Sciences Ltd
- Catalent Inc
- Boehringer Ingelheim Group
- Recipharm AB
- Baxter Biopharma Solutions (Baxter International Inc)
Key Milestones in NA Pharmaceutical CMO Market Industry
- 2020: Increased demand for CMO services due to the COVID-19 pandemic.
- 2021: Several significant M&A deals reshaping the market landscape.
- 2022: Focus on supply chain resilience and diversification.
- 2023: Increased investment in digitalization and automation within the industry.
Strategic Outlook for NA Pharmaceutical CMO Market Market
The NA Pharmaceutical CMO market presents a robust outlook, driven by continued pharmaceutical innovation and the increasing demand for outsourced manufacturing services. Strategic opportunities exist for CMOs focused on delivering high-quality, cost-effective solutions, embracing technological advancements, and fostering collaborative partnerships to address evolving customer needs. Companies that proactively adapt to changing regulations, invest in sustainable manufacturing practices, and prioritize data-driven decision-making are best positioned for success in this dynamic market.
NA Pharmaceutical CMO Market Segmentation
-
1. Service Type
-
1.1. Active P
- 1.1.1. Small Molecule
- 1.1.2. Large Molecule
- 1.1.3. High Potency API (HPAPI)
-
1.2. Finished
- 1.2.1. Solid Dose Formulation
- 1.2.2. Liquid Dose Formulation
- 1.2.3. Injectable Dose Formulation
- 1.3. Secondary Packaging
-
1.1. Active P
NA Pharmaceutical CMO Market Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
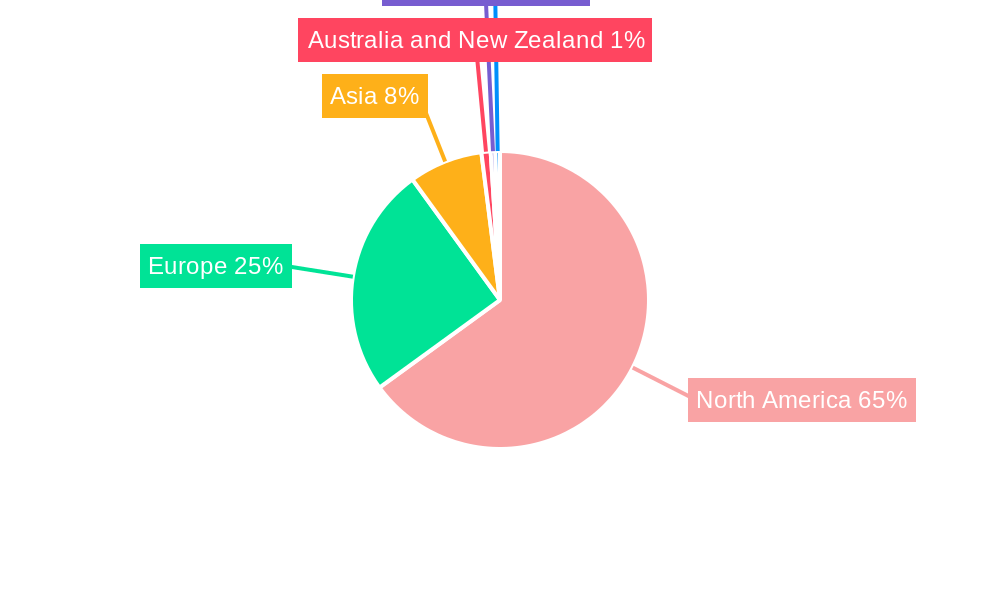
NA Pharmaceutical CMO Market Regional Market Share

Geographic Coverage of NA Pharmaceutical CMO Market
NA Pharmaceutical CMO Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 5.20% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. ; Growing emphasis on drug discovery and outsourcing of manufacturing; Strong R&D Investments
- 3.3. Market Restrains
- 3.3.1. Increasing Lead Time and Logistics Costs; Stringent Regulatory Requirements; Capacity Utilization Issues Affecting the Profitability of CMOs
- 3.4. Market Trends
- 3.4.1. Finished Dosage Formulation (FDF) Development and Manufacturing is Expected to Witness Significant Growth
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global NA Pharmaceutical CMO Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Service Type
- 5.1.1. Active P
- 5.1.1.1. Small Molecule
- 5.1.1.2. Large Molecule
- 5.1.1.3. High Potency API (HPAPI)
- 5.1.2. Finished
- 5.1.2.1. Solid Dose Formulation
- 5.1.2.2. Liquid Dose Formulation
- 5.1.2.3. Injectable Dose Formulation
- 5.1.3. Secondary Packaging
- 5.1.1. Active P
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. North America
- 5.1. Market Analysis, Insights and Forecast - by Service Type
- 6. Competitive Analysis
- 6.1. Global Market Share Analysis 2025
- 6.2. Company Profiles
- 6.2.1 Thermo Fisher Scientific Inc (Patheon Inc )
- 6.2.1.1. Overview
- 6.2.1.2. Products
- 6.2.1.3. SWOT Analysis
- 6.2.1.4. Recent Developments
- 6.2.1.5. Financials (Based on Availability)
- 6.2.2 Siegfried AG*List Not Exhaustive
- 6.2.2.1. Overview
- 6.2.2.2. Products
- 6.2.2.3. SWOT Analysis
- 6.2.2.4. Recent Developments
- 6.2.2.5. Financials (Based on Availability)
- 6.2.3 Aenova Group
- 6.2.3.1. Overview
- 6.2.3.2. Products
- 6.2.3.3. SWOT Analysis
- 6.2.3.4. Recent Developments
- 6.2.3.5. Financials (Based on Availability)
- 6.2.4 Lonza Group AG
- 6.2.4.1. Overview
- 6.2.4.2. Products
- 6.2.4.3. SWOT Analysis
- 6.2.4.4. Recent Developments
- 6.2.4.5. Financials (Based on Availability)
- 6.2.5 Pfizer CentreSource (Pfizer Inc )
- 6.2.5.1. Overview
- 6.2.5.2. Products
- 6.2.5.3. SWOT Analysis
- 6.2.5.4. Recent Developments
- 6.2.5.5. Financials (Based on Availability)
- 6.2.6 AbbVie Inc
- 6.2.6.1. Overview
- 6.2.6.2. Products
- 6.2.6.3. SWOT Analysis
- 6.2.6.4. Recent Developments
- 6.2.6.5. Financials (Based on Availability)
- 6.2.7 Jubilant Life Sciences Ltd
- 6.2.7.1. Overview
- 6.2.7.2. Products
- 6.2.7.3. SWOT Analysis
- 6.2.7.4. Recent Developments
- 6.2.7.5. Financials (Based on Availability)
- 6.2.8 Catalent Inc
- 6.2.8.1. Overview
- 6.2.8.2. Products
- 6.2.8.3. SWOT Analysis
- 6.2.8.4. Recent Developments
- 6.2.8.5. Financials (Based on Availability)
- 6.2.9 Boehringer Ingelheim Group
- 6.2.9.1. Overview
- 6.2.9.2. Products
- 6.2.9.3. SWOT Analysis
- 6.2.9.4. Recent Developments
- 6.2.9.5. Financials (Based on Availability)
- 6.2.10 Recipharm AB
- 6.2.10.1. Overview
- 6.2.10.2. Products
- 6.2.10.3. SWOT Analysis
- 6.2.10.4. Recent Developments
- 6.2.10.5. Financials (Based on Availability)
- 6.2.11 Baxter Biopharma Solutions (Baxter International Inc )
- 6.2.11.1. Overview
- 6.2.11.2. Products
- 6.2.11.3. SWOT Analysis
- 6.2.11.4. Recent Developments
- 6.2.11.5. Financials (Based on Availability)
- 6.2.1 Thermo Fisher Scientific Inc (Patheon Inc )
List of Figures
- Figure 1: Global NA Pharmaceutical CMO Market Revenue Breakdown (Million, %) by Region 2025 & 2033
- Figure 2: North America NA Pharmaceutical CMO Market Revenue (Million), by Service Type 2025 & 2033
- Figure 3: North America NA Pharmaceutical CMO Market Revenue Share (%), by Service Type 2025 & 2033
- Figure 4: North America NA Pharmaceutical CMO Market Revenue (Million), by Country 2025 & 2033
- Figure 5: North America NA Pharmaceutical CMO Market Revenue Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global NA Pharmaceutical CMO Market Revenue Million Forecast, by Service Type 2020 & 2033
- Table 2: Global NA Pharmaceutical CMO Market Revenue Million Forecast, by Region 2020 & 2033
- Table 3: Global NA Pharmaceutical CMO Market Revenue Million Forecast, by Service Type 2020 & 2033
- Table 4: Global NA Pharmaceutical CMO Market Revenue Million Forecast, by Country 2020 & 2033
- Table 5: United States NA Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 6: Canada NA Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 7: Mexico NA Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the NA Pharmaceutical CMO Market?
The projected CAGR is approximately 5.20%.
2. Which companies are prominent players in the NA Pharmaceutical CMO Market?
Key companies in the market include Thermo Fisher Scientific Inc (Patheon Inc ), Siegfried AG*List Not Exhaustive, Aenova Group, Lonza Group AG, Pfizer CentreSource (Pfizer Inc ), AbbVie Inc, Jubilant Life Sciences Ltd, Catalent Inc, Boehringer Ingelheim Group, Recipharm AB, Baxter Biopharma Solutions (Baxter International Inc ).
3. What are the main segments of the NA Pharmaceutical CMO Market?
The market segments include Service Type .
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
; Growing emphasis on drug discovery and outsourcing of manufacturing; Strong R&D Investments.
6. What are the notable trends driving market growth?
Finished Dosage Formulation (FDF) Development and Manufacturing is Expected to Witness Significant Growth.
7. Are there any restraints impacting market growth?
Increasing Lead Time and Logistics Costs; Stringent Regulatory Requirements; Capacity Utilization Issues Affecting the Profitability of CMOs.
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 4950, and USD 6800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "NA Pharmaceutical CMO Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the NA Pharmaceutical CMO Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the NA Pharmaceutical CMO Market?
To stay informed about further developments, trends, and reports in the NA Pharmaceutical CMO Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
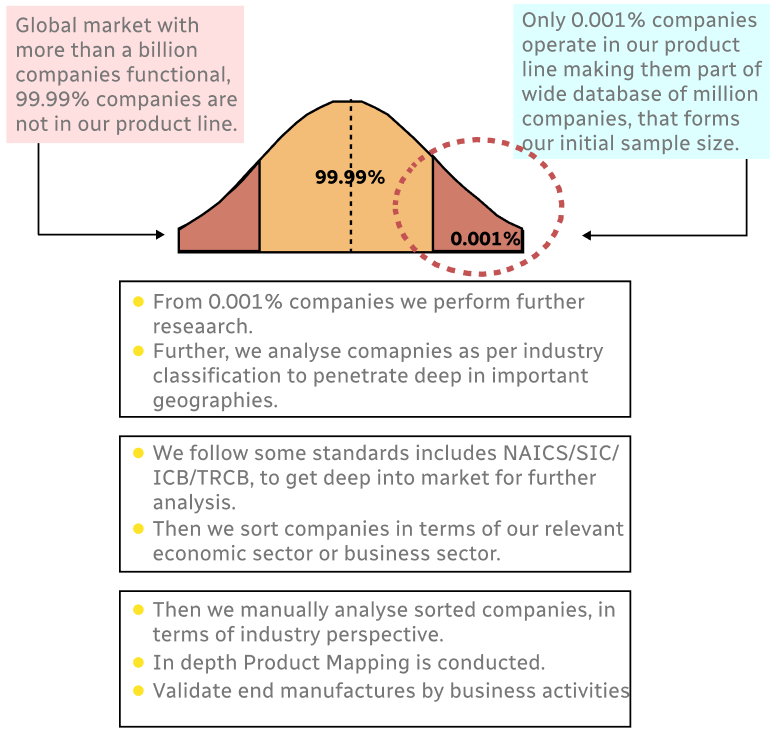
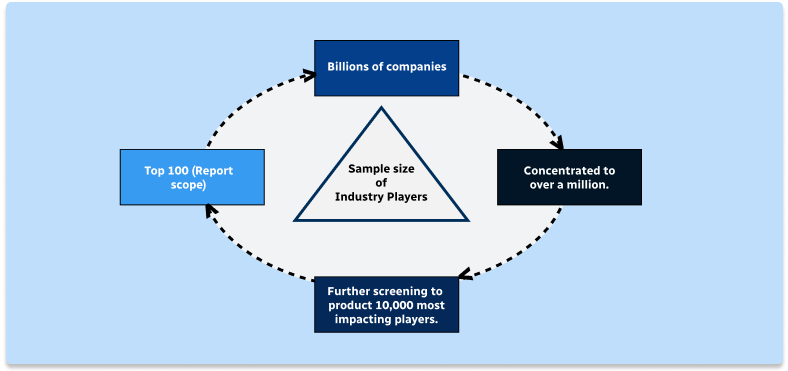
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
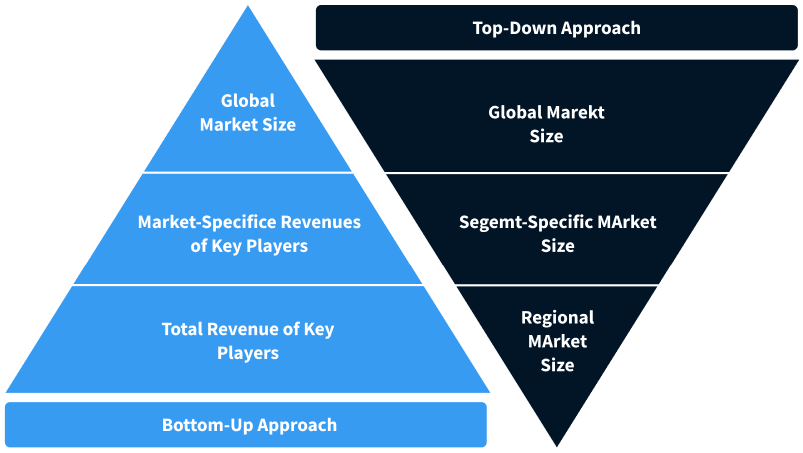
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
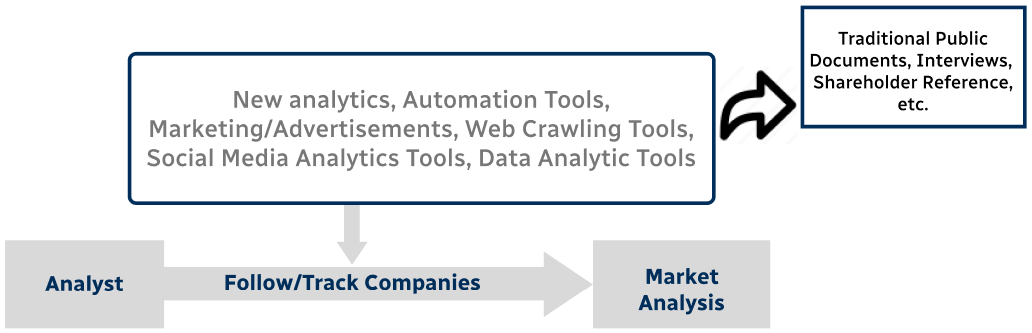
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


