Key Insights
The global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) market is experiencing robust growth, projected to reach \$243.29 million in 2025 and maintain a Compound Annual Growth Rate (CAGR) of 6.41% from 2025 to 2033. This expansion is driven by several key factors. The increasing complexity of drug development, coupled with rising R&D costs, compels pharmaceutical and biotechnology companies to outsource aspects of their drug development and manufacturing processes to specialized CDMOs. This allows them to focus on core competencies, accelerate time-to-market, and mitigate risks associated with in-house production. Furthermore, the burgeoning demand for biopharmaceuticals, advanced therapies (like cell and gene therapies), and personalized medicines is fueling the growth of the CDMO market. The market is segmented by research phase (pre-clinical through Phase IV) and service type (API manufacturing, HPAPI, various dosage formulations, and secondary packaging), reflecting the diverse needs of the pharmaceutical industry. The substantial presence of established players like Thermo Fisher Scientific, Lonza, and Catalent, alongside emerging players, underscores the market's competitive yet collaborative landscape. Significant regional variations exist, with North America and Europe likely holding the largest market shares initially, followed by a rapid increase in the Asia-Pacific region due to increasing investment in pharmaceutical manufacturing and a rising middle class.
-Market.png)
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Market Size (In Million)
The market's growth trajectory will be influenced by several trends. Technological advancements in drug manufacturing processes, including automation and digitalization, will improve efficiency and reduce costs. A growing focus on sustainability and environmental considerations within the pharmaceutical industry is likely to drive demand for CDMOs offering environmentally friendly manufacturing processes. Regulatory changes and approvals across different geographical regions will continue to play a significant role, influencing market access and adoption of new technologies and therapies. However, challenges such as maintaining consistent quality control across outsourced manufacturing processes and managing intellectual property rights remain important considerations for both CDMOs and their clients. Future market dominance will likely depend on CDMOs’ ability to adapt quickly to emerging trends, embrace technological innovations, and maintain high ethical and quality standards.
-Market.png)
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Company Market Share
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market: A Comprehensive Report (2019-2033)
This comprehensive report provides a detailed analysis of the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) market, offering invaluable insights for industry stakeholders, investors, and strategic decision-makers. Covering the period from 2019 to 2033, with a focus on 2025, this report delves into market dynamics, key trends, leading players, and future growth prospects. The global market size is projected to reach xx Million by 2033, exhibiting a CAGR of xx% during the forecast period (2025-2033).
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Concentration & Dynamics
The Pharmaceutical Contract Development and Manufacturing Organization (CDMO) market exhibits a moderately concentrated landscape, with a few large players holding significant market share. However, the presence of numerous smaller, specialized CDMOs contributes to a dynamic competitive environment. Market share data indicates that the top 5 players account for approximately xx% of the global market, while the remaining share is distributed amongst numerous smaller firms. This concentration is driven by economies of scale, technological advancements, and global reach. The innovation ecosystem is vibrant, with ongoing research and development focused on advanced technologies like cell and gene therapies, leading to continuous product and service improvements. Stringent regulatory frameworks, particularly concerning GMP (Good Manufacturing Practices), influence operational costs and market entry barriers. While substitute products are limited due to the highly specialized nature of CDMO services, the market experiences competitive pressure from companies offering integrated services along the drug development value chain. End-user trends emphasize a growing preference for outsourcing, driven by cost optimization, access to specialized expertise, and speed-to-market pressures.
M&A activity in the CDMO sector has been significant over the past few years, with xx major deals recorded between 2019 and 2024. These mergers and acquisitions aim to expand service offerings, geographic reach, and technological capabilities.
- Market Concentration: Top 5 players hold approximately xx% market share.
- M&A Activity: xx major deals recorded from 2019 to 2024.
- Regulatory Landscape: Stringent GMP regulations influence market dynamics.
- Innovation: Focus on advanced therapies (cell & gene) fuels competition.
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Industry Insights & Trends
The Pharmaceutical CDMO market is experiencing robust growth, driven by several key factors. The increasing outsourcing of drug development and manufacturing activities by pharmaceutical companies is a major driver, reducing capital expenditure and operational burden. The rising prevalence of chronic diseases globally fuels the demand for new therapies, leading to increased outsourcing of the development and manufacturing processes to specialized CDMOs. The market's expansion is also propelled by technological advancements, including automation, AI-driven processes, and continuous manufacturing, improving efficiency and quality. These innovations significantly impact overall cost and timelines, making CDMO services attractive for pharmaceutical clients. Consumer behavior plays a vital role as patients increasingly demand access to innovative and effective therapies, pushing for accelerated drug development and quicker access to markets. The market’s growth trajectory reflects these underlying drivers, with a substantial increase in the number of new drug approvals and the launch of innovative treatment modalities. The global market value reached xx Million in 2024, demonstrating the rapid expansion.
Key Markets & Segments Leading Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market
The North American region currently dominates the Pharmaceutical CDMO market, holding the largest market share, followed by Europe and Asia-Pacific. This dominance is attributed to several factors:
- North America: Strong pharmaceutical R&D base, robust regulatory frameworks, and high spending on healthcare.
- Europe: Established pharmaceutical industry, presence of leading CDMO players, and growing demand for innovative therapies.
- Asia-Pacific: Rapidly expanding pharmaceutical sector, increasing outsourcing trend, and cost-effective manufacturing capabilities.
Drivers of Market Growth (By Region):
- Economic Growth: Strong economic performance in key regions fuels healthcare spending.
- Infrastructure Development: Modernized infrastructure supports CDMO operations.
- Government Initiatives: Supportive policies promoting pharmaceutical R&D.
Dominant Segments:
- By Research Phase CRO Segment: Phase III clinical trials currently represent the largest segment, driven by the significant scale of late-stage clinical development.
- By Service Type CMO Segment: Active Pharmaceutical Ingredient (API) manufacturing and finished dosage formulation (FDF) development and manufacturing are the most significant segments, reflecting the high demand for both core components and final products in the drug production pipeline. Solid dosage formulations (tablets and other formats) maintain a significant share owing to the prevalent demand for oral medications.
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Product Developments
Recent years have witnessed significant innovation in CDMO services, with a focus on improving efficiency, quality, and speed. The integration of advanced technologies such as continuous manufacturing, AI-driven process optimization, and automated quality control systems has enhanced productivity and reduced manufacturing costs. The development of specialized capabilities in handling high-potency APIs and complex drug formulations has broadened the scope of services offered. This evolution not only attracts new clients but also strengthens the competitive positions of leading CDMO companies.
Challenges in the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Market
The Pharmaceutical CDMO market faces several challenges, including stringent regulatory compliance requirements which demand substantial investments in quality control and assurance. Supply chain disruptions and the availability of raw materials can impact production schedules and costs. Intense competition among CDMO companies necessitates continuous innovation and efficiency improvements to maintain a competitive edge. The capacity constraints for highly specialized services, such as cell and gene therapy manufacturing, contribute to price pressures. These factors can collectively impact profitability and market growth.
Forces Driving Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Growth
Several factors are driving the growth of the CDMO market:
- Technological advancements: Automation, AI, and continuous manufacturing enhance efficiency.
- Economic factors: Increasing healthcare expenditure fuels demand for new therapies.
- Regulatory changes: Streamlined approval processes incentivize drug development.
- Strategic partnerships: Collaboration between CDMOs and pharmaceutical companies.
Long-Term Growth Catalysts in the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market
Long-term growth will be propelled by ongoing investments in advanced technologies, strategic partnerships for expanding capacity and service offerings, and the expansion of services into emerging markets. Continued innovation in drug delivery systems and advanced therapies will fuel demand. The focus on personalized medicine will further enhance the need for flexible and specialized CDMO capabilities.
Emerging Opportunities in Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market
Emerging opportunities lie in the expansion of services into cell and gene therapy manufacturing, the adoption of digital technologies for enhanced efficiency, and the provision of integrated services across the drug development lifecycle. Focus on sustainable manufacturing practices and environmentally friendly processes is gaining traction. There’s also substantial potential in emerging markets with growing pharmaceutical sectors.
Leading Players in the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Sector
- LSK Global Pharma Service Co Ltd
- Pharmaceutical Product Development LLC (Thermo Fisher Scientific Inc)
- Hangzhou Tigermed Consulting Co Ltd
- Famar SA
- PAREXEL International Corporation
- CMIC Holdings Co Ltd
- PRA Health Sciences Inc (Icon PLC)
- Lonza Group
- Pfizer CentreSource
- SGS Life Science Services SA
- Samsung Bioepis Co Ltd
- Jubilant Pharmova Ltd
- WuXi AppTec Inc
- Tesa Labtec GmbH (TESA SE)
- Patheon Inc (Thermo Fisher Scientific Inc)
- Syneos Health Inc
- IQVIA Holdings Inc
- ARX LLC
- Tapemark
- Catalent Inc
- Boehringer Ingelheim Group
- Novotech Pty Ltd
- LabCorp Drug Development
- Aenova Holding GmbH
- Recipharm AB
- Sagimet Biosciences (3V Biosciences Inc)
- Baxter Biopharma Solutions (Baxter International Inc)
- Quanticate Ltd
Key Milestones in Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Industry
- January 2024: FAMAR and Lavipharm announce a new collaboration, expanding FAMAR's European presence.
- January 2024: Pluri launches "pluriCDMO," a new cell therapy manufacturing division with a 47,000-ft2 GMP facility.
- October 2023: IQVIA and Argenx collaborate on innovative pharmacovigilance services for rare autoimmune diseases.
Strategic Outlook for Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Market
The future of the Pharmaceutical CDMO market holds immense potential. Continued investment in advanced technologies and capacity expansion will be crucial. Strategic alliances and partnerships will play a significant role in expanding service offerings and global reach. A focus on sustainable and environmentally conscious manufacturing practices will further enhance the attractiveness of CDMO services to clients and stakeholders. The market's long-term prospects remain strong, driven by the increasing demand for innovative therapies and the ongoing trend of outsourcing drug development and manufacturing.
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Segmentation
-
1. Service Type CMO Segment
-
1.1. Active P
- 1.1.1. Small Molecule
- 1.1.2. Large Molecule
- 1.1.3. High Potency (HPAPI)
-
1.2. Finished
-
1.2.1. Solid Dose Formulation
- 1.2.1.1. Tablets
- 1.2.1.2. Others (Capsules, Powders, etc.)
- 1.2.2. Liquid Dose Formulation
- 1.2.3. Injectable Dose Formulation
-
1.2.1. Solid Dose Formulation
- 1.3. Secondary Packaging
-
1.1. Active P
-
2. Research Phase CRO Segment
- 2.1. Pre-clinical
- 2.2. Phase I
- 2.3. Phase II
- 2.4. Phase III
- 2.5. Phase IV
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
-
2. Europe
- 2.1. United Kingdom
- 2.2. Germany
- 2.3. France
- 2.4. Italy
-
3. Asia
- 3.1. China
- 3.2. India
- 3.3. Japan
- 3.4. Australia and New Zealand
-
4. Latin America
- 4.1. Brazil
- 4.2. Mexico
- 4.3. Argentina
-
5. Middle East and Africa
- 5.1. United Arab Emirates
- 5.2. Saudi Arabia
- 5.3. South Africa
- 6. North America
- 7. Europe
- 8. Asia
- 9. Australia and New Zealand
- 10. Latin America
- 11. Middle East and Africa
-Market.png)
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Regional Market Share
Geographic Coverage of Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 6.41% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Outsourcing Volume by Big Pharmaceutical Companies; Advent of CDMO Model into the Market; Increasing Investment in R&D
- 3.3. Market Restrains
- 3.3.1. Increasing Lead Time and Logistics Costs; Stringent Regulatory Requirements; Capacity Utilization Issues Affecting the Profitability of CMOs
- 3.4. Market Trends
- 3.4.1. Increasing Investment in R&D Drives the Market
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 5.1.1. Active P
- 5.1.1.1. Small Molecule
- 5.1.1.2. Large Molecule
- 5.1.1.3. High Potency (HPAPI)
- 5.1.2. Finished
- 5.1.2.1. Solid Dose Formulation
- 5.1.2.1.1. Tablets
- 5.1.2.1.2. Others (Capsules, Powders, etc.)
- 5.1.2.2. Liquid Dose Formulation
- 5.1.2.3. Injectable Dose Formulation
- 5.1.2.1. Solid Dose Formulation
- 5.1.3. Secondary Packaging
- 5.1.1. Active P
- 5.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 5.2.1. Pre-clinical
- 5.2.2. Phase I
- 5.2.3. Phase II
- 5.2.4. Phase III
- 5.2.5. Phase IV
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. Europe
- 5.3.3. Asia
- 5.3.4. Latin America
- 5.3.5. Middle East and Africa
- 5.3.6. North America
- 5.3.7. Europe
- 5.3.8. Asia
- 5.3.9. Australia and New Zealand
- 5.3.10. Latin America
- 5.3.11. Middle East and Africa
- 5.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 6. North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 6.1.1. Active P
- 6.1.1.1. Small Molecule
- 6.1.1.2. Large Molecule
- 6.1.1.3. High Potency (HPAPI)
- 6.1.2. Finished
- 6.1.2.1. Solid Dose Formulation
- 6.1.2.1.1. Tablets
- 6.1.2.1.2. Others (Capsules, Powders, etc.)
- 6.1.2.2. Liquid Dose Formulation
- 6.1.2.3. Injectable Dose Formulation
- 6.1.2.1. Solid Dose Formulation
- 6.1.3. Secondary Packaging
- 6.1.1. Active P
- 6.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 6.2.1. Pre-clinical
- 6.2.2. Phase I
- 6.2.3. Phase II
- 6.2.4. Phase III
- 6.2.5. Phase IV
- 6.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 7. Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 7.1.1. Active P
- 7.1.1.1. Small Molecule
- 7.1.1.2. Large Molecule
- 7.1.1.3. High Potency (HPAPI)
- 7.1.2. Finished
- 7.1.2.1. Solid Dose Formulation
- 7.1.2.1.1. Tablets
- 7.1.2.1.2. Others (Capsules, Powders, etc.)
- 7.1.2.2. Liquid Dose Formulation
- 7.1.2.3. Injectable Dose Formulation
- 7.1.2.1. Solid Dose Formulation
- 7.1.3. Secondary Packaging
- 7.1.1. Active P
- 7.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 7.2.1. Pre-clinical
- 7.2.2. Phase I
- 7.2.3. Phase II
- 7.2.4. Phase III
- 7.2.5. Phase IV
- 7.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 8. Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 8.1.1. Active P
- 8.1.1.1. Small Molecule
- 8.1.1.2. Large Molecule
- 8.1.1.3. High Potency (HPAPI)
- 8.1.2. Finished
- 8.1.2.1. Solid Dose Formulation
- 8.1.2.1.1. Tablets
- 8.1.2.1.2. Others (Capsules, Powders, etc.)
- 8.1.2.2. Liquid Dose Formulation
- 8.1.2.3. Injectable Dose Formulation
- 8.1.2.1. Solid Dose Formulation
- 8.1.3. Secondary Packaging
- 8.1.1. Active P
- 8.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 8.2.1. Pre-clinical
- 8.2.2. Phase I
- 8.2.3. Phase II
- 8.2.4. Phase III
- 8.2.5. Phase IV
- 8.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 9. Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 9.1.1. Active P
- 9.1.1.1. Small Molecule
- 9.1.1.2. Large Molecule
- 9.1.1.3. High Potency (HPAPI)
- 9.1.2. Finished
- 9.1.2.1. Solid Dose Formulation
- 9.1.2.1.1. Tablets
- 9.1.2.1.2. Others (Capsules, Powders, etc.)
- 9.1.2.2. Liquid Dose Formulation
- 9.1.2.3. Injectable Dose Formulation
- 9.1.2.1. Solid Dose Formulation
- 9.1.3. Secondary Packaging
- 9.1.1. Active P
- 9.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 9.2.1. Pre-clinical
- 9.2.2. Phase I
- 9.2.3. Phase II
- 9.2.4. Phase III
- 9.2.5. Phase IV
- 9.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 10. Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 10.1.1. Active P
- 10.1.1.1. Small Molecule
- 10.1.1.2. Large Molecule
- 10.1.1.3. High Potency (HPAPI)
- 10.1.2. Finished
- 10.1.2.1. Solid Dose Formulation
- 10.1.2.1.1. Tablets
- 10.1.2.1.2. Others (Capsules, Powders, etc.)
- 10.1.2.2. Liquid Dose Formulation
- 10.1.2.3. Injectable Dose Formulation
- 10.1.2.1. Solid Dose Formulation
- 10.1.3. Secondary Packaging
- 10.1.1. Active P
- 10.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 10.2.1. Pre-clinical
- 10.2.2. Phase I
- 10.2.3. Phase II
- 10.2.4. Phase III
- 10.2.5. Phase IV
- 10.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 11. North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 11.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 11.1.1. Active P
- 11.1.1.1. Small Molecule
- 11.1.1.2. Large Molecule
- 11.1.1.3. High Potency (HPAPI)
- 11.1.2. Finished
- 11.1.2.1. Solid Dose Formulation
- 11.1.2.1.1. Tablets
- 11.1.2.1.2. Others (Capsules, Powders, etc.)
- 11.1.2.2. Liquid Dose Formulation
- 11.1.2.3. Injectable Dose Formulation
- 11.1.2.1. Solid Dose Formulation
- 11.1.3. Secondary Packaging
- 11.1.1. Active P
- 11.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 11.2.1. Pre-clinical
- 11.2.2. Phase I
- 11.2.3. Phase II
- 11.2.4. Phase III
- 11.2.5. Phase IV
- 11.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 12. Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 12.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 12.1.1. Active P
- 12.1.1.1. Small Molecule
- 12.1.1.2. Large Molecule
- 12.1.1.3. High Potency (HPAPI)
- 12.1.2. Finished
- 12.1.2.1. Solid Dose Formulation
- 12.1.2.1.1. Tablets
- 12.1.2.1.2. Others (Capsules, Powders, etc.)
- 12.1.2.2. Liquid Dose Formulation
- 12.1.2.3. Injectable Dose Formulation
- 12.1.2.1. Solid Dose Formulation
- 12.1.3. Secondary Packaging
- 12.1.1. Active P
- 12.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 12.2.1. Pre-clinical
- 12.2.2. Phase I
- 12.2.3. Phase II
- 12.2.4. Phase III
- 12.2.5. Phase IV
- 12.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 13. Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 13.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 13.1.1. Active P
- 13.1.1.1. Small Molecule
- 13.1.1.2. Large Molecule
- 13.1.1.3. High Potency (HPAPI)
- 13.1.2. Finished
- 13.1.2.1. Solid Dose Formulation
- 13.1.2.1.1. Tablets
- 13.1.2.1.2. Others (Capsules, Powders, etc.)
- 13.1.2.2. Liquid Dose Formulation
- 13.1.2.3. Injectable Dose Formulation
- 13.1.2.1. Solid Dose Formulation
- 13.1.3. Secondary Packaging
- 13.1.1. Active P
- 13.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 13.2.1. Pre-clinical
- 13.2.2. Phase I
- 13.2.3. Phase II
- 13.2.4. Phase III
- 13.2.5. Phase IV
- 13.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 14. Australia and New Zealand Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 14.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 14.1.1. Active P
- 14.1.1.1. Small Molecule
- 14.1.1.2. Large Molecule
- 14.1.1.3. High Potency (HPAPI)
- 14.1.2. Finished
- 14.1.2.1. Solid Dose Formulation
- 14.1.2.1.1. Tablets
- 14.1.2.1.2. Others (Capsules, Powders, etc.)
- 14.1.2.2. Liquid Dose Formulation
- 14.1.2.3. Injectable Dose Formulation
- 14.1.2.1. Solid Dose Formulation
- 14.1.3. Secondary Packaging
- 14.1.1. Active P
- 14.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 14.2.1. Pre-clinical
- 14.2.2. Phase I
- 14.2.3. Phase II
- 14.2.4. Phase III
- 14.2.5. Phase IV
- 14.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 15. Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 15.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 15.1.1. Active P
- 15.1.1.1. Small Molecule
- 15.1.1.2. Large Molecule
- 15.1.1.3. High Potency (HPAPI)
- 15.1.2. Finished
- 15.1.2.1. Solid Dose Formulation
- 15.1.2.1.1. Tablets
- 15.1.2.1.2. Others (Capsules, Powders, etc.)
- 15.1.2.2. Liquid Dose Formulation
- 15.1.2.3. Injectable Dose Formulation
- 15.1.2.1. Solid Dose Formulation
- 15.1.3. Secondary Packaging
- 15.1.1. Active P
- 15.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 15.2.1. Pre-clinical
- 15.2.2. Phase I
- 15.2.3. Phase II
- 15.2.4. Phase III
- 15.2.5. Phase IV
- 15.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 16. Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 16.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 16.1.1. Active P
- 16.1.1.1. Small Molecule
- 16.1.1.2. Large Molecule
- 16.1.1.3. High Potency (HPAPI)
- 16.1.2. Finished
- 16.1.2.1. Solid Dose Formulation
- 16.1.2.1.1. Tablets
- 16.1.2.1.2. Others (Capsules, Powders, etc.)
- 16.1.2.2. Liquid Dose Formulation
- 16.1.2.3. Injectable Dose Formulation
- 16.1.2.1. Solid Dose Formulation
- 16.1.3. Secondary Packaging
- 16.1.1. Active P
- 16.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 16.2.1. Pre-clinical
- 16.2.2. Phase I
- 16.2.3. Phase II
- 16.2.4. Phase III
- 16.2.5. Phase IV
- 16.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 17. Competitive Analysis
- 17.1. Global Market Share Analysis 2025
- 17.2. Company Profiles
- 17.2.1 LSK Global Pharma Service Co Ltd
- 17.2.1.1. Overview
- 17.2.1.2. Products
- 17.2.1.3. SWOT Analysis
- 17.2.1.4. Recent Developments
- 17.2.1.5. Financials (Based on Availability)
- 17.2.2 Pharmaceutical Product Development LLC (Thermo Fisher Scientific Inc )
- 17.2.2.1. Overview
- 17.2.2.2. Products
- 17.2.2.3. SWOT Analysis
- 17.2.2.4. Recent Developments
- 17.2.2.5. Financials (Based on Availability)
- 17.2.3 Hangzhou Tigermed Consulting Co Ltd
- 17.2.3.1. Overview
- 17.2.3.2. Products
- 17.2.3.3. SWOT Analysis
- 17.2.3.4. Recent Developments
- 17.2.3.5. Financials (Based on Availability)
- 17.2.4 Famar SA
- 17.2.4.1. Overview
- 17.2.4.2. Products
- 17.2.4.3. SWOT Analysis
- 17.2.4.4. Recent Developments
- 17.2.4.5. Financials (Based on Availability)
- 17.2.5 PAREXEL International Corporation
- 17.2.5.1. Overview
- 17.2.5.2. Products
- 17.2.5.3. SWOT Analysis
- 17.2.5.4. Recent Developments
- 17.2.5.5. Financials (Based on Availability)
- 17.2.6 CMIC Holdings Co Ltd
- 17.2.6.1. Overview
- 17.2.6.2. Products
- 17.2.6.3. SWOT Analysis
- 17.2.6.4. Recent Developments
- 17.2.6.5. Financials (Based on Availability)
- 17.2.7 PRA Health Sciences Inc (Icon PLC)
- 17.2.7.1. Overview
- 17.2.7.2. Products
- 17.2.7.3. SWOT Analysis
- 17.2.7.4. Recent Developments
- 17.2.7.5. Financials (Based on Availability)
- 17.2.8 Lonza Group
- 17.2.8.1. Overview
- 17.2.8.2. Products
- 17.2.8.3. SWOT Analysis
- 17.2.8.4. Recent Developments
- 17.2.8.5. Financials (Based on Availability)
- 17.2.9 Pfizer CentreSource
- 17.2.9.1. Overview
- 17.2.9.2. Products
- 17.2.9.3. SWOT Analysis
- 17.2.9.4. Recent Developments
- 17.2.9.5. Financials (Based on Availability)
- 17.2.10 SGS Life Science Services SA
- 17.2.10.1. Overview
- 17.2.10.2. Products
- 17.2.10.3. SWOT Analysis
- 17.2.10.4. Recent Developments
- 17.2.10.5. Financials (Based on Availability)
- 17.2.11 Samsung Bioepis Co Ltd
- 17.2.11.1. Overview
- 17.2.11.2. Products
- 17.2.11.3. SWOT Analysis
- 17.2.11.4. Recent Developments
- 17.2.11.5. Financials (Based on Availability)
- 17.2.12 Jubilant Pharmova Ltd
- 17.2.12.1. Overview
- 17.2.12.2. Products
- 17.2.12.3. SWOT Analysis
- 17.2.12.4. Recent Developments
- 17.2.12.5. Financials (Based on Availability)
- 17.2.13 WuXi AppTec Inc
- 17.2.13.1. Overview
- 17.2.13.2. Products
- 17.2.13.3. SWOT Analysis
- 17.2.13.4. Recent Developments
- 17.2.13.5. Financials (Based on Availability)
- 17.2.14 Tesa Labtec GmbH (TESA SE)
- 17.2.14.1. Overview
- 17.2.14.2. Products
- 17.2.14.3. SWOT Analysis
- 17.2.14.4. Recent Developments
- 17.2.14.5. Financials (Based on Availability)
- 17.2.15 Patheon Inc (Thermo Fisher Scientific Inc )
- 17.2.15.1. Overview
- 17.2.15.2. Products
- 17.2.15.3. SWOT Analysis
- 17.2.15.4. Recent Developments
- 17.2.15.5. Financials (Based on Availability)
- 17.2.16 Syneos Health Inc
- 17.2.16.1. Overview
- 17.2.16.2. Products
- 17.2.16.3. SWOT Analysis
- 17.2.16.4. Recent Developments
- 17.2.16.5. Financials (Based on Availability)
- 17.2.17 IQVIA Holdings Inc
- 17.2.17.1. Overview
- 17.2.17.2. Products
- 17.2.17.3. SWOT Analysis
- 17.2.17.4. Recent Developments
- 17.2.17.5. Financials (Based on Availability)
- 17.2.18 ARX LLC
- 17.2.18.1. Overview
- 17.2.18.2. Products
- 17.2.18.3. SWOT Analysis
- 17.2.18.4. Recent Developments
- 17.2.18.5. Financials (Based on Availability)
- 17.2.19 Tapemark
- 17.2.19.1. Overview
- 17.2.19.2. Products
- 17.2.19.3. SWOT Analysis
- 17.2.19.4. Recent Developments
- 17.2.19.5. Financials (Based on Availability)
- 17.2.20 Catalent Inc
- 17.2.20.1. Overview
- 17.2.20.2. Products
- 17.2.20.3. SWOT Analysis
- 17.2.20.4. Recent Developments
- 17.2.20.5. Financials (Based on Availability)
- 17.2.21 Boehringer Ingelheim Group
- 17.2.21.1. Overview
- 17.2.21.2. Products
- 17.2.21.3. SWOT Analysis
- 17.2.21.4. Recent Developments
- 17.2.21.5. Financials (Based on Availability)
- 17.2.22 Novotech Pty Ltd
- 17.2.22.1. Overview
- 17.2.22.2. Products
- 17.2.22.3. SWOT Analysis
- 17.2.22.4. Recent Developments
- 17.2.22.5. Financials (Based on Availability)
- 17.2.23 LabCorp Drug Development
- 17.2.23.1. Overview
- 17.2.23.2. Products
- 17.2.23.3. SWOT Analysis
- 17.2.23.4. Recent Developments
- 17.2.23.5. Financials (Based on Availability)
- 17.2.24 Aenova Holding GmbH
- 17.2.24.1. Overview
- 17.2.24.2. Products
- 17.2.24.3. SWOT Analysis
- 17.2.24.4. Recent Developments
- 17.2.24.5. Financials (Based on Availability)
- 17.2.25 Recipharm AB
- 17.2.25.1. Overview
- 17.2.25.2. Products
- 17.2.25.3. SWOT Analysis
- 17.2.25.4. Recent Developments
- 17.2.25.5. Financials (Based on Availability)
- 17.2.26 Sagimet Biosciences (3V Biosciences Inc )*List Not Exhaustive
- 17.2.26.1. Overview
- 17.2.26.2. Products
- 17.2.26.3. SWOT Analysis
- 17.2.26.4. Recent Developments
- 17.2.26.5. Financials (Based on Availability)
- 17.2.27 Baxter Biopharma Solutions (Baxter International Inc )
- 17.2.27.1. Overview
- 17.2.27.2. Products
- 17.2.27.3. SWOT Analysis
- 17.2.27.4. Recent Developments
- 17.2.27.5. Financials (Based on Availability)
- 17.2.28 Quanticate Ltd
- 17.2.28.1. Overview
- 17.2.28.2. Products
- 17.2.28.3. SWOT Analysis
- 17.2.28.4. Recent Developments
- 17.2.28.5. Financials (Based on Availability)
- 17.2.1 LSK Global Pharma Service Co Ltd
List of Figures
- Figure 1: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Breakdown (Million, %) by Region 2025 & 2033
- Figure 2: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 3: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 4: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 5: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 6: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 7: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 8: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 9: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 10: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 11: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 12: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 13: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 14: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 15: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 16: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 17: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 18: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 19: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 20: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 21: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 22: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 23: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 24: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 25: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 26: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 27: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 28: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 29: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 30: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 31: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 32: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 33: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 34: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 35: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 36: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 37: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 38: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 39: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 40: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 41: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 42: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 43: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 44: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 45: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 46: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 47: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 48: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 49: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 50: Australia and New Zealand Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 51: Australia and New Zealand Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 52: Australia and New Zealand Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 53: Australia and New Zealand Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 54: Australia and New Zealand Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 55: Australia and New Zealand Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 56: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 57: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 58: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 59: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 60: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 61: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 62: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 63: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 64: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 65: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 66: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 67: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 2: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 3: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Region 2020 & 2033
- Table 4: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 5: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 6: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 7: United States Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 8: Canada Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 9: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 10: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 11: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 12: United Kingdom Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 13: Germany Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 14: France Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 15: Italy Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 16: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 17: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 18: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 19: China Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 20: India Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 21: Japan Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 22: Australia and New Zealand Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 23: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 24: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 25: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 26: Brazil Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 27: Mexico Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 28: Argentina Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 29: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 30: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 31: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 32: United Arab Emirates Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 33: Saudi Arabia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 34: South Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 35: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 36: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 37: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 38: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 39: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 40: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 41: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 42: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 43: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 44: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 45: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 46: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 47: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 48: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 49: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 50: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 51: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 52: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market?
The projected CAGR is approximately 6.41%.
2. Which companies are prominent players in the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market?
Key companies in the market include LSK Global Pharma Service Co Ltd, Pharmaceutical Product Development LLC (Thermo Fisher Scientific Inc ), Hangzhou Tigermed Consulting Co Ltd, Famar SA, PAREXEL International Corporation, CMIC Holdings Co Ltd, PRA Health Sciences Inc (Icon PLC), Lonza Group, Pfizer CentreSource, SGS Life Science Services SA, Samsung Bioepis Co Ltd, Jubilant Pharmova Ltd, WuXi AppTec Inc, Tesa Labtec GmbH (TESA SE), Patheon Inc (Thermo Fisher Scientific Inc ), Syneos Health Inc, IQVIA Holdings Inc, ARX LLC, Tapemark, Catalent Inc, Boehringer Ingelheim Group, Novotech Pty Ltd, LabCorp Drug Development, Aenova Holding GmbH, Recipharm AB, Sagimet Biosciences (3V Biosciences Inc )*List Not Exhaustive, Baxter Biopharma Solutions (Baxter International Inc ), Quanticate Ltd.
3. What are the main segments of the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market?
The market segments include Service Type CMO Segment, Research Phase CRO Segment.
4. Can you provide details about the market size?
The market size is estimated to be USD 243.29 Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Outsourcing Volume by Big Pharmaceutical Companies; Advent of CDMO Model into the Market; Increasing Investment in R&D.
6. What are the notable trends driving market growth?
Increasing Investment in R&D Drives the Market.
7. Are there any restraints impacting market growth?
Increasing Lead Time and Logistics Costs; Stringent Regulatory Requirements; Capacity Utilization Issues Affecting the Profitability of CMOs.
8. Can you provide examples of recent developments in the market?
January 2024 - A new collaboration was announced jointly by FAMAR and Lavipharm, two leading pharmaceutical companies. FAMAR is a leading provider of development and manufacturing services for pharmaceutical and cosmetic products (CDMO) and one of the major CDMO players in Europe. Lavipharm is a research and development (R&D) company that manufactures, imports, sells, and distributes pharmaceuticals and healthcare products in Greece.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market?
To stay informed about further developments, trends, and reports in the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
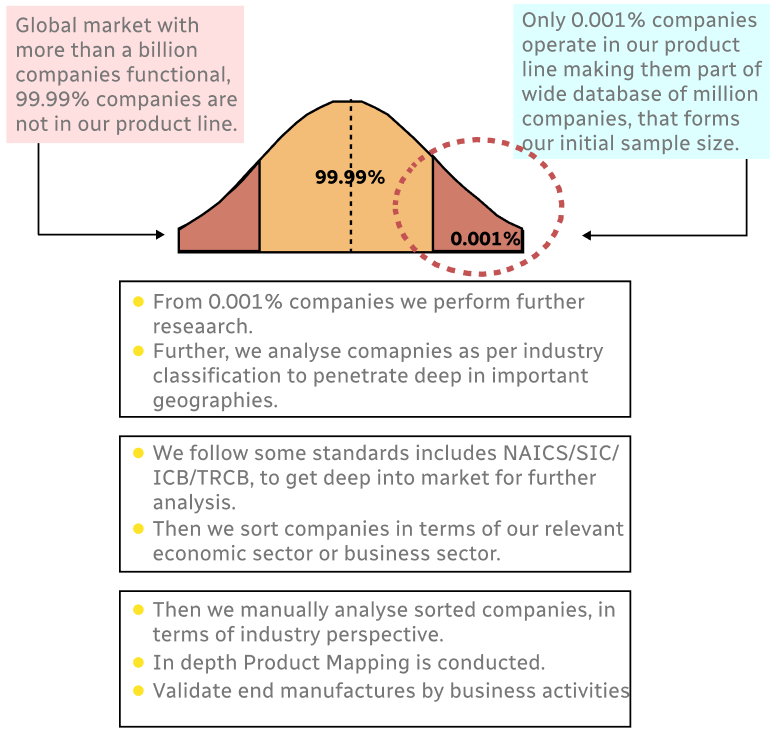
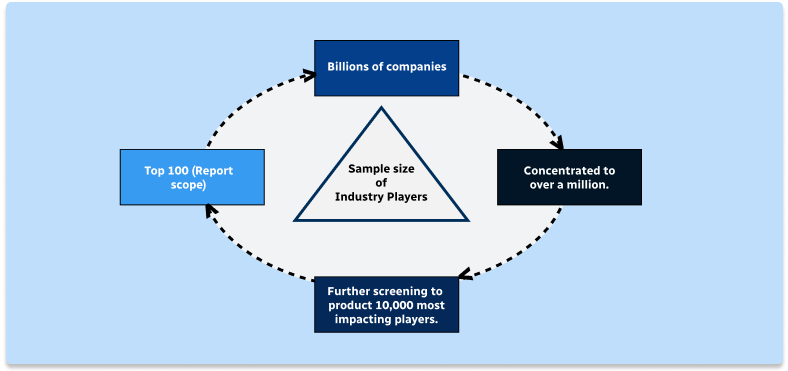
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
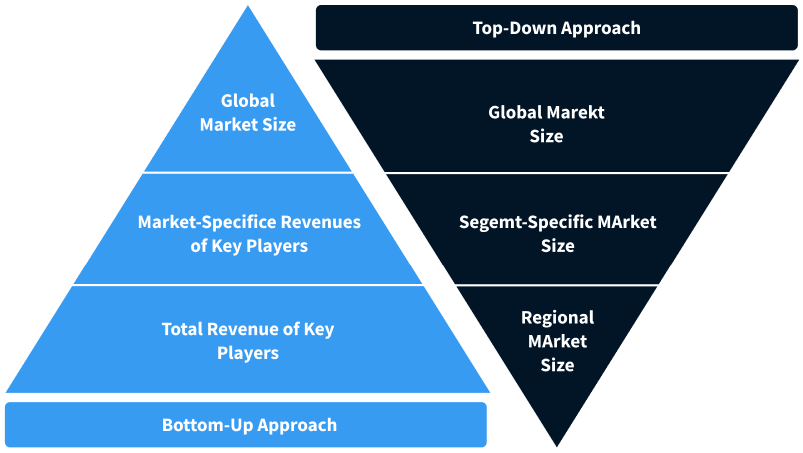
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
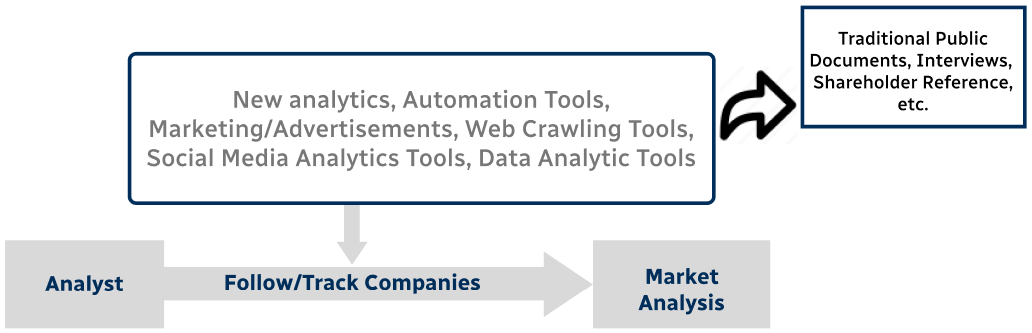
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


